
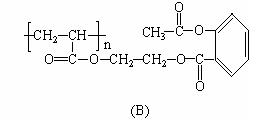
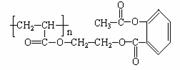
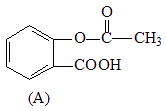 ��ѧ�Ұ�ҩ�������ڸ߷����������Ƴɻ��ͳ�Чҩ������˹ƥ���dz��õĽ�����ʹҩ���ṹ��ʽ��A������������ij�ۺ����ϣ��γɻ��ͳ�Ч��˹ƥ�֣�����һ�ֵĽṹΪB��
��ѧ�Ұ�ҩ�������ڸ߷����������Ƴɻ��ͳ�Чҩ������˹ƥ���dz��õĽ�����ʹҩ���ṹ��ʽ��A������������ij�ۺ����ϣ��γɻ��ͳ�Ч��˹ƥ�֣�����һ�ֵĽṹΪB��
��1����˹ƥ�ֵķ���ʽΪ_________�������еĺ��������ŵ�������____________��
��2�����ͳ�Ч��˹ƥ���к�����������_______����1mol���ͳ�Ч��˹ƥ�������뺬______mol NaOH���ʵ���Һ��Ӧ��
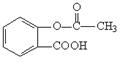
��3����д���밢˹ƥ�ַ��Ӿ�����ͬ�������ұ�����ֻ���������ȡ������ͬ���칹��Ľṹ��ʽ��д��2�����ɣ���
________________________________________________________________________��
��4����˹ƥ�ֿ��Ը�NaOH��Һ��Ӧ��д���÷�Ӧ�Ļ�ѧ����ʽ��
________________________________________________________________________��
��5��������û��ͳ�Ч��˹ƥ�ֺ������ڻ����ͷŰ�˹ƥ�ֵĻ�ѧ����ʽ�ǣ�
______________________________________________________________________��
��1��C9H8O4 �������Ȼ�
��2��3n 4n
 ��3��
��3��
��4��
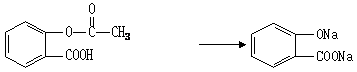 |
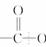
+ 3NaOH + CH3COONa+2H2O
��5��
 | |||
 | |||
![]()
![]() +nH2O n +
+nH2O n +

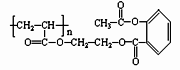
![]() + 2nH2O n +
+ 2nH2O n +
+ n HOCH2CH2OH �������б����а�˾ƥ�ַ��ӣ�
�����ص㿼�������������й����ŵ����ʺ���������ͬ���칹�����д��������ļ����ؼ����ǣ��ٻ��ͳ�Ч��˹ƥ��BΪ�߷��ӻ������ṹ�к�3n�������������е�һ������ˮ������֮һΪ�ӣ�Ҫ������NaOH��Ӧ������������4nmol NaOH��Ӧ�����밢˹ƥ�ַ��Ӿ�����ͬ�������ұ�����ֻ���������ȡ������ͬ���칹��Ľṹ��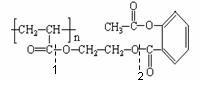 ʽ���ɹ̶���COOH��ֻ���� �������������ͬ���칹�塣��������û��ͳ�Ч��˹ƥ�ֺ������ڻ����ͷŰ�˹ƥ�ֵĹ��̿������������ˮ�ⷴӦ��Ҫ���а�˾ƥ�����ɣ�����Զϡ�2����������nmolH2O��������ͬʱ�ϡ�1��2����������ͼ��ʾ��������2nmolH2O����ѧ����ʽ��������д��ʽ��
ʽ���ɹ̶���COOH��ֻ���� �������������ͬ���칹�塣��������û��ͳ�Ч��˹ƥ�ֺ������ڻ����ͷŰ�˹ƥ�ֵĹ��̿������������ˮ�ⷴӦ��Ҫ���а�˾ƥ�����ɣ�����Զϡ�2����������nmolH2O��������ͬʱ�ϡ�1��2����������ͼ��ʾ��������2nmolH2O����ѧ����ʽ��������д��ʽ��


 ϰ�⾫ѡϵ�д�
ϰ�⾫ѡϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ѧ�Ұ�ҩ�������ڸ߷��������Ͽ��Ƴɻ��峤Чҩ������˾ƥ�ֵĽṹΪ��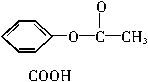 �������Խ���һ�ָ߾����������γɻ��ͳ�Чҩ�������е�һ�ֽṹ���£�
�������Խ���һ�ָ߾����������γɻ��ͳ�Чҩ�������е�һ�ֽṹ���£�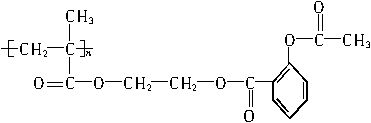
��1�����ͳ�Ч��˾ƥ������Ľṹ��ʽΪ____________________��
��2����ҩ����������Ի������ͷų���˾ƥ�֣�д���˹��̵Ļ�ѧ����ʽ____________________��
��3�����ָ߷����������ɵ���ۺ϶��õ��ģ�д���õ���Ľṹ��ʽ_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ���������ڸ߾����ϣ��γɻ��ͳ�Чҩ��������һ�ֵĽṹ��ʽΪ?
���������ڸ߾����ϣ��γɻ��ͳ�Чҩ��������һ�ֵĽṹ��ʽΪ?
�������������:
(1)���ͳ�Ч��˾ƥ������Ľṹ��ʽΪ___________��
(2)��ҩ����������ͨ��ˮ�����ÿ��Ի����ͷŰ�˾ƥ�֣��仯ѧ����ʽΪ___________��
(3)ԭ�߷������������___________ (д�ṹ��ʽ)�����Ӿ۷�Ӧ�õ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��������������һ�߾��������ϣ��γɻ��ͳ�Чҩ��������һ�ֵĽṹ��ʽΪ��
��������������һ�߾��������ϣ��γɻ��ͳ�Чҩ��������һ�ֵĽṹ��ʽΪ��
������������⣺
��1�����ͳ�Ч��˾ƥ�ֵ�����Ľṹ��ʽΪ________________��
��2����ҩ����������ͨ��ˮ�����ã����Ի������ͷų���˾ƥ�֣�д�����ˮ�ⷴӦ�Ļ�ѧ����ʽ��______________________________��
��3�����ָ߷����������ɵ��巢���ۺϷ�Ӧ�õ��ġ�д������Ľṹ��ʽ_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ѧ�Ұ�ҩ�������ڸ߷��������Ͽ��Ƴɻ��ͳ�Чҩ����˹ƥ�֣����ĽṹΪ��
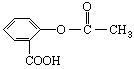
�����Խ���һ�ָ߾����������γɻ��ͳ�Чҩ�������е�һ�ֽṹ���£�
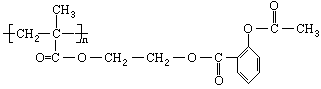
(1)д������Ľṹ��ʽ_____��
(2)��ҩ����������Ի������ͷų���˹ƥ�֣�д���˹��̵Ļ�ѧ����ʽ��____��
(3)���ָ߷�������ĵ���ĽṹΪ_____��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com