
���������ĿƼ�ʷר����Լɪ��ʿ��֤˵�����й������ھ��3 000����ǰ�����Ѿ�ʹ�ò����ˡ��������йز�����˵������ȷ����(����)
A������ͨ������ԭ����Ҫ�Ǵ��ʯ��ʯ��ʯӢ
B����ͨ�����ijɷ���Ҫ�ǹ����ơ�����ƺͶ�������
C�������ڼ����ۻ�ʱ�й̶����۵�
D��ʢ���ռ���Һ���Լ�ƿ�����ò���������Ϊ�˷�ֹ�ռ�������������ɹ����ƶ�ʹƿ����ƿ��ճ��һ��
 �������Ͽ��㱾ϵ�д�
�������Ͽ��㱾ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и�����������Һ�мȿ��Դ������棬�Ҽ�������������Һ��Ҳ��������������(����)
A��Na����Ba2����Cl����SO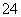 B��K����Na����NO
B��K����Na����NO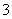 ��OH��
��OH��
C��H����NH ��Fe3����SO
��Fe3����SO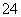 D��H����Cl����CH3COO����NO
D��H����Cl����CH3COO����NO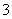
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵���У���ȷ����(����)
A�������Ħ�����ԼΪ22.4 L��mol��1
B��1 mol H2��������2 g������ռ�������22.4 L��mol��1
C���ڱ�״���£�1 mol�κ�������ռ�������ԼΪ22.4 L��mol��1
D���ڱ�״���£�1 mol�κ�������ռ�������ԼΪ22.4 L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
4.8 g O2��0.2 mol CO2�����ǵ����ʵ���֮����________������֮����________����ͬ��ͬѹ�µ����֮����________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������۷ֱ����������Һ��,�ܷų��� ��,�ҿ��ܴ����������(����)
��,�ҿ��ܴ����������(����)
A.H+��Ba2+��Cl-��N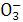 B.N
B.N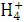 ��C
��C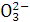 ��N
��N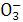 ��Na+
��Na+
C.N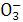 ��K+��[Al(OH)4]-��OH- D.Na+��Ba2+��Mg2+��HC
��K+��[Al(OH)4]-��OH- D.Na+��Ba2+��Mg2+��HC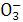
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��Һ�м��������ܲ����������ڸ���Һ��һ���ܴ����������������(����)
A��Na����K����Cl����SO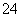 B��Cu2����Fe2����NO
B��Cu2����Fe2����NO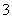 ��SO
��SO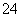
C��Na����Ca2����Cl����ClO�� D��K����NH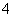 ��Cl����SO
��Cl����SO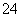
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����һ����Ҫ�Ľ������������������о���������Ҫ����;����ͼ�Ǵ����������Ʊ����Ĺ������̣�
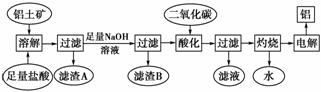
��֪�� ���������Ҫ�ɷ���Al2O3�������������SiO2��Fe2O3�����ʣ��ش��������⣺
(1)���������м�������������˺���������A����Ҫ�ɷ�Ϊ________��
(2)�ڹ��������У�������������������Һ�����з�����Ӧ�����ӷ���ʽΪ__________________________________________________________________________��
���˺���������B�ijɷ�Ϊ________���ö�����̼�ữ�����������Һ�е�������________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ͼʾ�У�AΪһ�ֳ����ĵ��ʣ�B��C��D��E�Ǻ���AԪ�صij����������֪A�ڿ�����ȼ�����ɵ���ɫ����(���ֲ�������ȥ)��
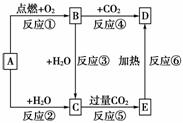
����д���пհף�
(1)д����ѧʽ��
A��________��B.________��C.________��D.________��
(2)���Ϸ�Ӧ�������û���Ӧ����________(��д���)��
(3)д������ת���Ļ�ѧ����ʽ��
A��C_______________________________________________________________��
B��D_______________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵����,����ȷ����(����)
A.����(H3PO4)��Ħ��������6.02��1023��������ӵ���������ֵ�����
B.6.02��1023�������Ӻ�6.02��1023������ӵ������ȵ���14��1
C.32 g����������ԭ����ĿΪ2��6.02��1023
D.���³�ѹ��,0.5��6.02��1023��һ����̼������ռ�������11.2 L
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com