


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A�� ��ͼ���ڷų�������Ȼ�̼��Һ |
B��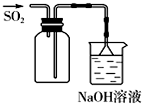 ��ͼ����ʵ�����ռ�SO2 |
C�� ��ͼ����ʵ�����Ʊ�Fe��OH��2 |
D��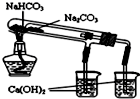 ��ͼ���ڱȽ�NaHCO3��Na2CO3���ȶ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� |
| ���� |
| A�����Ӽ����������ʽ�С�H���� |
| B������ˮ���������������ˮ������ת���� |
| C�������¶ȣ��淴Ӧ������������Ӧ���ʼ��� |
| D��������ϵѹǿ���÷�Ӧ�Ļ�ѧƽ�ⳣ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��������ʽ��NaHB��ˮ��Һ��ˮ�⣺HB-+H2O�TH3O++B2- | ||||
B����ⱥ��ʳ��ˮ��C1-+2H2O
| ||||
| C������ȼ�ϵ���ڼ��Խ����е�������Ӧʽ��O2+2H2O+4e-=4OH- | ||||
| D��FeBr2��Һ��ͨ�����Cl2��2Fe2++2Br-+2Cl2=2Fe3++Br2+4Cl- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����������Һ��ͨ������CO2��2C6H5O-+CO2+H2O��2C6H5OH+CO32- | ||
B����ȩ��Һ�м���������������Һ�����ȣ�HCHO+2[Ag��NH3��2]++2OH-
| ||
C����ȩ�����������ͭ����Һ��Ϻ���������ڣ�CH3CHO+2Cu��OH��2+OH-
| ||
| D����С�մ���Һ�м�����CO32-+2CH3COOH�TCO2��+H2O+2CH3COO- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
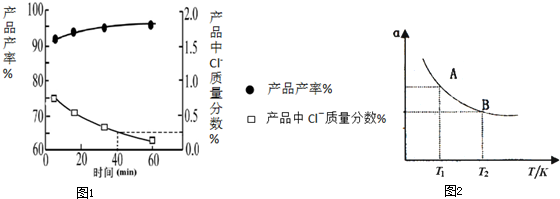
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�������������϶����������� |
| B�����������������ݣ�������ʹʪ��ĵ⻯�ص�����ֽ���� |
| C����Һ�����˻���ɫ���ǣ�������˺��ɫ |
| D��������δ�������ݣ������̪�Լ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com