
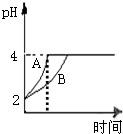
ГЃЮТЯТЃЌНЋa1mLb1mol?L-1CH3COOHШмвКМгШыЕНa2mLb2mol?L-1NaOHШмвКЁЃЯТСаНсТлжае§ШЗЕФЪЧЃЈЁЁЁЁЃЉ
AЃЎШєa1=a2ЃЌb1=b2ЃЌдђЛьКЯШмвКжаc(CH3COO-)=c(Na+)>c(H2)=c(OH-)
BЃЎШєa1=a2ЃЌb1=2b2ЃЌдђЛьКЯШмвКжаc(CH3COO-)>c(CH3COOH) >c(Na+)
CЃЎШєa1b1> a2b2ЃЌдђЛьКЯвКЕФpHвЛЖЈДѓгк7
DЃЎa1=a2ЃЌЧвЛьКЯШмвКЕФpH<7ЃЌдђb1вЛЖЈДѓгкb2

| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
 ЃЈ1ЃЉAЃЎNH3 BЃЎSO3 CЃЎCl2 DЃЎBaSO4 EЃЎОЦОЋ FЃЎCH3COONH4
ЃЈ1ЃЉAЃЎNH3 BЃЎSO3 CЃЎCl2 DЃЎBaSO4 EЃЎОЦОЋ FЃЎCH3COONH4ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
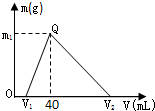 ГЃЮТЯТЃЌНЋвЛЖЈСПЕФФЦТСКЯН№жУгкЫЎжаЃЌКЯН№ШЋВПШмНтЃЌЕУЕН200mL CЃЈOH-ЃЉ=0.1mol/LЕФШмвКЃЌШЛКѓж№ЕЮМгШы1mol/L ЕФбЮЫсЃЌВтЕУЩњГЩГСЕэЕФжЪСПmгыЯћКФбЮЫсЕФЬхЛ§VЙиЯЕШчЭМЫљЪОЃЌдђЯТСаЫЕЗЈе§ШЗЕФЪЧЃЈЁЁЁЁЃЉ
ГЃЮТЯТЃЌНЋвЛЖЈСПЕФФЦТСКЯН№жУгкЫЎжаЃЌКЯН№ШЋВПШмНтЃЌЕУЕН200mL CЃЈOH-ЃЉ=0.1mol/LЕФШмвКЃЌШЛКѓж№ЕЮМгШы1mol/L ЕФбЮЫсЃЌВтЕУЩњГЩГСЕэЕФжЪСПmгыЯћКФбЮЫсЕФЬхЛ§VЙиЯЕШчЭМЫљЪОЃЌдђЯТСаЫЕЗЈе§ШЗЕФЪЧЃЈЁЁЁЁЃЉВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
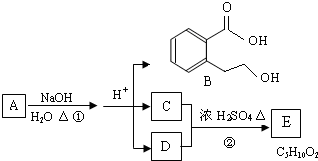
| ХЈСђЫс |
| МгШШ |
| ХЈСђЫс |
| МгШШ |


 аДГіЫФепжЎвЛМДПЩ
аДГіЫФепжЎвЛМДПЩ аДГіЫФепжЎвЛМДПЩ
аДГіЫФепжЎвЛМДПЩ| ЪЕбщБрКХ | CЮяжЪЕФСПХЈЖШЃЈmol?L-1ЃЉ | NaOHЮяжЪЕФСПХЈЖШЃЈmol?L-1ЃЉ | ЛьКЯШмвКЕФpH |
| m | 0.1 | 0.1 | pH=9 |
| n | 0.2 | 0.1 | pHЃМ7 |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ВщПДД№АИКЭНтЮі>>
АйЖШжТаХ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com