
��14�֣�
�����£���֪HCl��Һ��NaOH��Һ�����ζ���������ͼ��ʾ��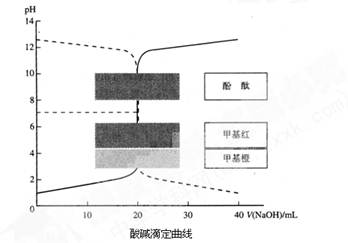
��1����һ������NaOH��Һ�еμ�HCl��Һ������Ϊͼ�� ���ʵ�ߡ������ߡ�����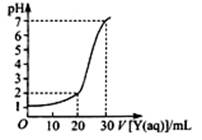
��2����ͼΪ��10mLһ�����ʵ���Ũ�ȵ�NaHSO4��ҺX��һ�����ʵ���Ũ�ȵ�����������ҺY�ζ���ͼ������ͼ���Ƴ�X��Y�����ʵ���Ũ�ȷֱ��� �� ��
��3��ij�о���ѧϰС����о����⣺ʳ������������g/100mL���IJⶨ�����ǽ������µζ�������
A��ȡijƷ�ư״�25.00mL���� �����������ƣ��У�������ˮϡ��10����
B���� �����������ƣ���ȡϡ�ͺ�İ״���Һ20.00mL������250mL��ƿ�У����� ����ָʾ�����ƣ�1��2�Ρ�
C����0.05 mol��L?1NaOH����Һ�ζ������յ㡣���³�ʼ���յ������
��ע�⣺�ζ��ظ�����3�Ρ���
�������ϲ�������������ش��������⡣
�ٲ�����C���У��ζ�ʱ������ע�� ���յ������� ��
�������������в�������ʹ�ⶨ���ƫ�ߵ��� ��
a��ϡ�Ͱ״�����ˮԤ��δ������д���
b��ʢNaOH��Һ�ļ�ʽ�ζ���δ�ñ�Һ��ϴ
c���ζ�ǰ������ȷ���ζ��յ�ʱ���Ӷ���
d���ӽ��յ�ʱ������������ˮϴ����ƿ
�������С���������Ʒ���̱�����ע��һ�£����ܵ�ԭ��֮һ�� ��
��14�֣�
��1������ ��2�֣� ��2��0.09 mol��L?1 0.03 mol��L?1 ��ÿ��2�֣�
��3����A��250mL����ƿ��1�֣�
��B����ʽ�ζ��ܣ�1�֣�����̪��Һ��1�֣�
����ƿ����Һ��ɫ�ı仯����1�֣�
��Һ����ɫ��Ϊdz��ɫ������30s�ڲ���ɫ����1�֣�
��a��b��2�֣�
��ʳ��ϡ��ʱ��������Ʋ�����ʵ��ϡ�ͱ��������������ͱ�����������ȡ���ƫС���ζ��յ���ɫ����30s��ȥ���ζ��������ڼ�ʽ�ζ��ܵļ��촦�����ݣ������̼�ˮ����ϡ�͵ȡ���1�֣�
����


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
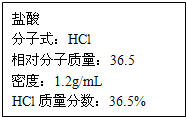 ��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺
��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺| 1000Vd |
| 22400+36.5V |
| 1000Vd |
| 22400+36.5V |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ͭ����������ʹ�õĽ��������㷺Ӧ���ڵ������Ṥ����е���������ҵ�ȣ���֪Cu2O��H2SO4�ܷ�����Ӧ��Cu2O+H2SO4=Cu+CuSO4+H2O��
ͭ����������ʹ�õĽ��������㷺Ӧ���ڵ������Ṥ����е���������ҵ�ȣ���֪Cu2O��H2SO4�ܷ�����Ӧ��Cu2O+H2SO4=Cu+CuSO4+H2O��
| ||
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com