ijʵ��С����0.50mol?L
-1 NaOH��Һ��0.55mol?L
-1������Һ�����к��ȵIJⶨ��������0.50mol?L
-1 NaOH��Һ
��1����ʵ����Ҫʹ��250mL NaOH��Һ��������Ҫ����NaOH����
g��
��2������250mL0.50mol?L
-1 NaOH��������Ҫ�IJ�������������ĸ����
��
A����Ͳ B���ձ� C�������� D����ͷ�ι� E��250mL����ƿ
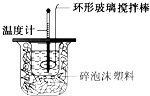
�ⶨϡ�����ϡ���������к��ȵ�ʵ��װ����ͼ��ʾ��
��1��д���÷�Ӧ���Ȼ�ѧ����ʽ���к���Ϊ57.3kJ?mol
-1����
��
��2��ȡ50mL NaOH��Һ��50mL������Һ����ʵ�飬�ش��������⣮
��ʵ������У�����ĭ���ϵ�������
�ڽ�����Ϊ0.50mol?L
-1 NaOH��Һ��0.55mol?L
-1������Һ���ܶȶ���1g?cm
-3���кͺ�������Һ�ı�����c=4.18J?g
-1?��
-1���Ҳ���¶ȵ�ƽ������ֵ��3.4�棬������к��ȡ�H=
kJ?mol
-1 ��ȡС�����һλ����
������ʵ����ֵ�����57.3kJ?mol
-1��ƫ����ƫ����ܵ�ԭ������ǣ�����ĸ��
��
a��ʵ��װ�ñ��¡�����Ч����
b���ֶ�ΰ�������Һ����ʢ���������Ƶ�С�ձ���
c�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�Ӳⶨ������Һ���¶�
d������������Һ����װ�����ձ����м���������Һ
�������60mL0.5mol?L
-1����������Һ��50mL0.55mol?L
-1������Һ���з�Ӧ��������ʵ����ȣ����ų�������
�����ȡ�����ȡ�����������к���
�����ȡ�����ȡ������ٶ����������ʵ�������ȫ��ͬ��




 ��ҵ����CO2��H2��һ�������������·�Ӧ�ϳɼ״����ų��������ȣ�CO2��g��+3H2��g��?CH3OH��g��+��H2O��g����H1 �ش��������⣮
��ҵ����CO2��H2��һ�������������·�Ӧ�ϳɼ״����ų��������ȣ�CO2��g��+3H2��g��?CH3OH��g��+��H2O��g����H1 �ش��������⣮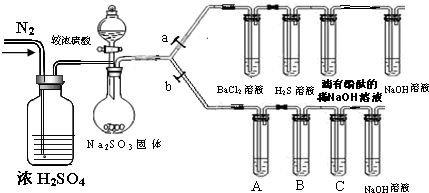
 ijʵ��С����0.50mol?L-1 NaOH��Һ��0.55mol?L-1������Һ�����к��ȵIJⶨ��������0.50mol?L-1 NaOH��Һ
ijʵ��С����0.50mol?L-1 NaOH��Һ��0.55mol?L-1������Һ�����к��ȵIJⶨ��������0.50mol?L-1 NaOH��Һ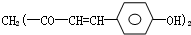 ���Ƴɵ���ֽ���Լ�������ԣ��ܹ���1mol�û�������Ӧ��NaOH��H2��������ֱ�Ϊ��������
���Ƴɵ���ֽ���Լ�������ԣ��ܹ���1mol�û�������Ӧ��NaOH��H2��������ֱ�Ϊ��������