
����Ŀ������п����п������Ҫ�Ļ�������ԭ�ϡ�
��1��ZnO �� Al2O3 �Ļ�ѧ�������ƣ�ZnO �� NaOH ��Һ��ת����[Zn(OH)4]2�����ӷ���ʽΪ_____________��
��2������п�õ�������п�к���Ǧ��ͭ�����ʣ��ᴿ�������£�
![]()
![]()
����ͼ�еġ������Ϊ________���ѧʽ����
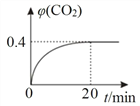
��ij�¶�ʱ���ڷ�Ӧ��ķ�Ӧ¯�У���ʼʱ c(CO)Ϊ 0.3 molL1����Ӧ������ CO2 ��������� ��(CO2)��ͼ��ʾ����Ӧ���ƽ�ⳣ�� K��_____��
�����д�ʩ��������߷�Ӧ���� ZnO ת���ʵ���________��
a������ ZnO ��Ͷ���� b���ʵ���ѹ c����п������ʱ����
�ܷ�Ӧ���У�ÿת�� 1mol ���ӣ���Ӧ���� 174 kJ���� H2��_____________��
��3���ⶨ����п��Ʒ���ȣ���ȡ 0.5000g ��Ʒ�����ܺ����� 250 mL ����ƿ�У�ҡ�ȡ���ȡ 25.00 mL ����Һ���� 0.04000 molL1 �� EDTA��Na2H2Y����Һ�ζ����е� Zn2+����Ӧ����ʽΪ Zn2+��H2Y2��ZnY2��2H+�����ʲ���Ӧ����ƽ�еζ����Σ�ƽ������ EDTA ��Һ 15.12mL��
�����ζ���δ�� EDTA ��Һ��ϴ���ⶨ�����___���ƫ�ߡ�����ƫ�͡����䡱����
����Ʒ����Ϊ��________________���г�����ʽ���ɣ���
��4���ʵ�ӫ�����е���ɫӫ��ۺ��� ZnS�������� 0.05mol ZnS ��ӫ������� 500mL�����У���ȫ�ܽ����Һ�� c(S2)��__________ molL1������֪��Ksp(ZnS)��2.5��1023��������Һ����ı仯��
���𰸡� ZnO+2OH-+H2O=[Zn(OH)4]2- Zn 0.4mol/L c -696kJ/mol ƫ�� ![]() 2.5��10-22
2.5��10-22
��������(1)ZnO��Al2O3�Ļ�ѧ�������ƣ����������������Ʒ�Ӧ����ƫ�����ƣ���������п���������Ƶķ�Ӧ�ķ���ʽΪ��ZnO+H2O+2OH-=[Zn(OH)4]2-���ʴ�Ϊ��ZnO+H2O+2OH-=[Zn(OH)4]2-��
(2)����Ӧ����ZnO(s)+CO(g)Zn(g)+CO2(g)��п��������Ϊ�����������Ϊ����п���ʴ�Ϊ��Zn��
��ij�¶�ʱ���ڷ�Ӧ���ķ�Ӧ¯�У���ʼʱc(CO)Ϊ0.3molL-1����Ӧ�����дﵽƽ��CO2�����������(CO2)��ͼ��ʾΪ0.4��
ZnO(s)+CO(g)Zn(g)+CO2(g)
��ʼ��(mol/L) 0.3 0
�仯��(mol/L) x x
ƽ����(mol/L) 0.3-x x
![]() =0.4��x=0.12��ƽ�ⳣ��K=
=0.4��x=0.12��ƽ�ⳣ��K=![]() =0.67���ʴ�Ϊ��0.67��
=0.67���ʴ�Ϊ��0.67��
��a������ZnO��Ͷ����������пΪ���岻Ӱ��ƽ�⣬����пת���ʲ��䣬��a����b����Ӧǰ������������䣬�ʵ���ѹ����Ӱ��ƽ���ƶ�����b����c����п������ʱ���룬ƽ�������������пת��������c��ȷ���ʴ�Ϊ��c��
����Ӧ����2Zn(g)+O2(g)�T2ZnO(s)����Ӧ��2molZn��ȫ��Ӧ����ת��4mol����Ӧÿת��1mol���ӣ���Ӧ����174kJ��ת��4mol���ӷ�Ӧ����696KJ����Ӧ�ʱ���H=-696KJ/mol���ʴ�Ϊ��-696KJ/mol��
(3)���ζ���δ��EDTA��Һ��ϴ���ڲ�ˮĤ��ϡ�ͱ���Һ�����ı���Һ��������ⶨ���ƫ�ߣ��ʴ�Ϊ��ƫ�ߣ�
����ȡ0.5000g��Ʒ�����ܺ�����250mL����ƿ�У�ҡ�ȣ���ȡ25.00mL����Һ����0.04000molL-1��EDTA(Na2H2Y)��Һ�ζ����е�Zn2+(��Ӧ����ʽΪZn2++H2Y2-�TZnY2-+2H+�����ʲ���Ӧ)��ƽ�еζ����Σ�ƽ������EDTA��Һ15.12mL��
Zn2++H2Y2-�TZnY2-+2H+��
1 1
n 15.12��10-3L��0.04000mol/L
n(ZnO)=n(Zn2+)=15.12��10-3L��0.04000mol/L��250mL��Һ��n(ZnO)=15.12��10-3L��0.04000mol/L��![]() ����Ʒ����=
����Ʒ����=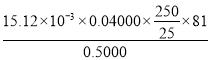 ��100%���ʴ�Ϊ��
��100%���ʴ�Ϊ��  ��100%��
��100%��
(4)������0.05molZnS��ӫ�������500mL�����У���ȫ�ܽ����Һ��п����Ũ��c(Zn2+)=![]() =0.1mol/L��Ksp(ZnS)=2.5��10-23=c(Zn2+)c(S2-)��
=0.1mol/L��Ksp(ZnS)=2.5��10-23=c(Zn2+)c(S2-)��
c(S2-)��2.5��10-22mol/L���ʴ�Ϊ��2.5��10-22��


 ��ѧ����ϵ�д�
��ѧ����ϵ�д� �ο�������ϵ�д�
�ο�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������¼����л��
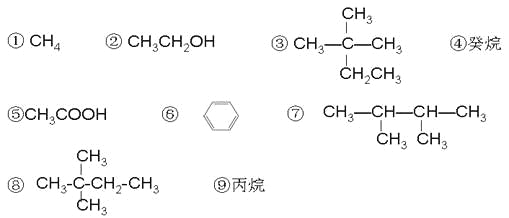
�������������������ʰ�Ҫ��ش��������⣺
��1����Է�������Ϊ44�������Ľṹ��ʽΪ ��
��2�������к���14����ԭ�ӵ������ķ���ʽ�� ��
��3��������Ϊͬ���칹����� ������ţ���
��4������������ζ��������ȡ�����л�����������������������Һ�巢��һȡ����Ӧ�Ļ�ѧ����ʽ ��
��5������������ʾ�٢ۢܢ��۷е�ߵ�˳�� ������ţ���
��6���л������ڼ��������º�CuO��Ӧ�Ļ�ѧ����ʽ ��
��7����120����1.01��105Pa�����£�ij����̬����������O2��ȫ��Ӧ��÷�Ӧǰ����������û�з����ı䣬������� ������ţ�����������Ϊ ��ϵ��
��8���л�����������һ�������·�����Ӧ�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������һ±���飬���ܷ�����ȥ��Ӧ���� �� ��
A. CH3CH2ClB. (CH3)2CHClC. (CH3)3CClD. (CH3)3CCH2Cl
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����е����Ų�ʽ�ǻ�̬ԭ�ӵ���( )
A.1s1
B.1s22s12p1
C.1s22s22p63s2
D.1s22s22p63p1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ܱ������зֱ����Ne��H2��O2�������壬�����ǵ��¶Ⱥ��ܶȶ���ͬʱ�������������ѹǿ��p���Ӵ�С��˳���ǣ� ��
A��p��H2����p��Ne����p��O2�� B��p��O2����p��Ne����p��H2��
C��p��H2����p��O2����p��Ne�� D��p��Ne����p��H2����p��O2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ȣ�ClO2���Ǽ�������ˮ�Ҳ���ˮ������ѧ��Ӧ�Ļ���ɫ���壬�е�Ϊ 11�棬�����ڴ��������ˮ��ijС����ʵ������̽�� ClO2 �� Na2S �ķ�Ӧ���ش��������⣺
��1��ClO2 ���Ʊ�
��֪��SO2��2NaClO3��H2SO4��2ClO2����2NaHSO4
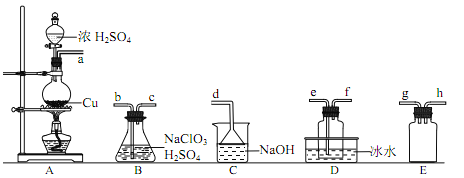
��װ�� A �з�Ӧ�Ļ�ѧ����ʽΪ____________��
�����ռ������ ClO2��ѡ����ͼ�е�װ�ã�������˳��Ϊ a��________________��������������Сд��ĸ��ʾ��
��װ�� D ��������__________________��
��2��ClO2 �� Na2S �ķ�Ӧ
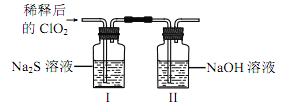
�������ռ����� ClO2 �� N2 ϡ������ǿ���ȶ��ԣ�����������ϡ�ͺ�� ClO2 ͨ����ͼ��ʾװ���г�ַ�Ӧ���õ���ɫ������Һ��һ��ʱ���ͨ������ʵ��̽�� I �з�Ӧ�IJ��
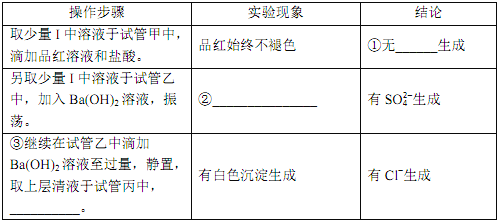
��___________����___________����___________��
��ClO2 �� Na2S ��Ӧ�����ӷ���ʽΪ____________�����ڴ��������ˮʱ��ClO2 �����Cl2 ���ŵ���____________����дһ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ij��̬ԭ�ӵ���Χ�����Ų�Ϊ3d14s2��������˵����ȷ����( )
A. ��Ԫ������P��B. ��Ԫ��ԭ�Ӻ�����4�����Ӳ�
C. ��Ԫ��ԭ������㹲��3������D. ��Ԫ��ԭ��M�ܲ㹲��8������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ܹ��ü��ܽ��͵���
�� A.�����Ļ�ѧ���ʱ������ȶ�
�� B.���³�ѹ�£����Һ�壬��Ϊ����
�� C.ϡ������һ����ѷ�����ѧ��Ӧ
�� D.�����ӷ��������ѻӷ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������(Ti)����Ϊ21���ͽ������䵥�ʺͻ�������й㷺��Ӧ�ü�ֵ��
��ش��������⣺
(1)Ti�Ļ�̬ԭ�Ӽ۵����Ų�ʽΪ______��
(2)����TiO2������������Ӧ�Ĵ�����

������ķ����в�ȡsp2��ʽ�ӻ���̼ԭ����_____________�������������в�ȡsp3��ʽ�ӻ���ԭ�Ӷ�Ӧ��Ԫ�صĵ縺���ɴ�С��˳��Ϊ___________________��
(3)��Ti3+�������Ļ�ѧʽΪ[TiCl(H2O)5]Cl2��H2O,���������к��еĻ�ѧ��������____________��1 mol��������к��е�![]() ����Ŀ��____________��
����Ŀ��____________��
(4)ͨ��X������֪̽KCl��MgO��CaO��TiN�ľ�����NaCl�ľ���ṹ���ơ���֪���� ���Ӿ���ľ������������£�

KCl��MgO��CaO��TiN�������Ӿ����۵��ɸߵ��͵�˳��Ϊ______________________��
(5)ij�ֵ����Ѿ���ľ�����ͼ��ʾ���þ�������Nԭ�Ӿ�������������Nԭ����_____����Tiԭ�ӵ���λ��Ϊ______������λԭ�ӹ��ɵĿռ乹��Ϊ_____���þ�����N��Tiԭ��֮����������Ϊa nm����õ����Ѿ�����ܶ�Ϊ______g��cm-3��NAΪ�����ӵ�������ֵ��ֻ�м���ʽ)��

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com