
ĻĀĶ¼ÖŠXŹĒĪŽÖ§Į“µÄ”¢¾ßÓŠ¹ūĻćĪ¶µÄŗĻ³ÉĻćĮĻ£¬æÉÓĆÓŚµ÷Å䶹ÖÖ¹ūĻćŠĶĻć¾«”£ŅŃÖŖ
DŌŚ±ź×¼×“æöĻĀµÄĆܶČĪŖ1.25 g/L£¬Ęä²śĮææÉŅŌÓĆĄ“ŗāĮæŅ»øö¹ś¼ŅŹÆÓĶ»Æ¹¤·¢Õ¹Ė®Ę½”£EŹĒÉś»ī
ÖŠ³£¼ūµÄŅ»ÖÖÓŠ»śĪļ”£ø÷ĪļÖŹ¼ä×Ŗ»Æ¹ŲĻµČēĻĀ£ŗ

Ēė»Ų“šĻĀĮŠĪŹĢā”£
(1)AµÄĆū³ĘŹĒ ”£
(2)BÖŠĖłŗ¬µÄ¹ŁÄÜĶÅŹĒ ”£
(3)C+E XµÄ»Æѧ·“Ó¦ĄąŠĶŹĒ ·“Ó¦”£
XµÄ»Æѧ·“Ó¦ĄąŠĶŹĒ ·“Ó¦”£
(4)Š“³öČĪŅāĮ½ÖÖÓėA¾ßÓŠĻąĶ¬¹ŁÄÜĶŵÄAµÄĶ¬·ÖŅģ¹¹ĢåµÄ½į¹¹¼ņŹ½ (²»ŗ¬A)£ŗ ”£
(5)XÓėĒāŃõ»ÆÄĘČÜŅŗ·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ ”£
(6)ŅŌDĪŖŌĮĻÉś²śŅ»ÖÖ³£¼ūĖÜĮĻµÄ»Æѧ·½³ĢŹ½ŹĒ ”£
£Ø1£©1£¶”“¼ £Ø2£©Č©»ł £Ø3£©õ„»Æ·“Ó¦£Ø»ņČ”“ś·“Ó¦£©
£Ø4£©(CH3)3COH”¢CH3CH2CH(OH)CH3”¢(CH3)2CHCH2OH
£Ø5£©CH3CH2CH2COOCH2CH3£«NaOH CH3CH2CH2COONa£«CH3CH2OH
CH3CH2CH2COONa£«CH3CH2OH
£Ø6£©
”¾½āĪö”æ
ŹŌĢā·ÖĪö£ŗDµÄ²śĮææÉŅŌÓĆĄ“ŗāĮæŅ»øö¹ś¼ŅŹÆÓĶ»Æ¹¤·¢Õ¹Ė®Ę½£¬ŌņDŹĒŅŅĻ©”£AŃõ»ÆÉś³ÉB£¬BŃõ»ÆÉś³ÉC£¬CŗĶE·“Ӧɜ³É¾ßÓŠ¹ūĻćĪ¶µÄŗĻ³ÉĻćĮĻ£¬ÕāĖµĆ÷XÓ¦øĆŹĒõ„Ąą£¬ĖłŅŌCŹĒōČĖį£¬EŹĒ“¼”£ÕāĖµĆ÷EŹĒŅŅĻ©ŗĶĖ®Ģįø߼ӳɷ“Ӧɜ³ÉµÄŅŅ“¼”£Ōņøł¾ŻŌ×ÓŹŲŗćæÉÖŖ£¬CÓ¦øĆŹĒ¶”Ėį”£ÓÉÓŚXŹĒĪŽÖ§Į“µÄ£¬ĖłŅŌBŹĒ¶”Č©£¬ŌņAŹĒÕż¶”“¼£¬¼“1£¶”“¼”£
æ¼µć£ŗæ¼²éÓŠ»śĪļĆū³Ę”¢¹ŁÄÜĶÅ”¢½į¹¹¼ņŹ½”¢Ķ¬·ÖŅģ¹¹ĢåµÄÅŠ¶ĻŅŌ¼°ÓŠ»ś·“Ó¦·½³ĢŹ½µÄŹéŠ“µČ
µćĘĄ£ŗøĆĢāŹĒøßæ¼ÖŠµÄ³£¼ūĢāŠĶ£¬ŹōÓŚÖŠµČÄѶȵďŌĢā”£ŹŌĢāĢł½üøßæ¼£¬»ł“”ŠŌĒ棬ŌŚ×¢ÖŲ¶Ōѧɜ»ł“”ÖŖŹ¶¹®¹ĢÓėѵĮ·µÄĶ¬Ź±£¬²ąÖŲ¶ŌѧɜÄÜĮ¦µÄÅąŃųÓė½āĢā·½·ØµÄÖøµ¼ŗĶѵĮ·”£øĆĢāµÄ¹Ų¼üŹĒ¼Ē×”³£¼ū¹ŁÄÜĶŵĽį¹¹”¢ŠŌÖŹŅŌ¼°¹ŁÄÜĶÅÖ®¼äµÄĻą»„×Ŗ»Æ£¬Č»ŗó½įŗĻĢāŅāĮé»īŌĖÓĆ¼“æÉ”£


 µŚ1¾ķµ„ŌŖŌĀæ¼ĘŚÖŠĘŚÄ©ĻµĮŠ“š°ø
µŚ1¾ķµ„ŌŖŌĀæ¼ĘŚÖŠĘŚÄ©ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

| ”÷ |
| ”÷ |


²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010Äź±±¾©ŹŠĪ÷³ĒĒųø߶žĻĀŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌĢā ĢāŠĶ£ŗĢīæÕĢā
(14·Ö) ĻĀĶ¼ÖŠXŹĒĪŽÖ§Į“µÄ”¢¾ßÓŠ¹ūĻćĪ¶µÄŗĻ³ÉĻćĮĻ£¬æÉÓĆÓŚµ÷Å䶹ÖÖ¹ūĻćŠĶĻć¾«”£ŅŃÖŖDŌŚ±ź×¼×“æöĻĀµÄĆܶČĪŖ1.25 g/L£¬Ęä²śĮææÉŅŌÓĆĄ“ŗāĮæŅ»øö¹ś¼ŅŹÆÓĶ»Æ¹¤·¢Õ¹Ė®Ę½”£EŹĒÉś»īÖŠ³£¼ūµÄŅ»ÖÖÓŠ»śĪļ”£ø÷ĪļÖŹ¼ä×Ŗ»Æ¹ŲĻµČēĻĀ£ŗ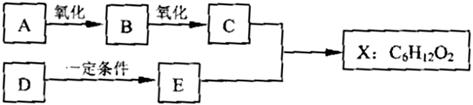
Ēė»Ų“šĻĀĮŠĪŹĢā”£
£Ø1£©AµÄĆū³ĘŹĒ ”£
£Ø2£©BÖŠĖłŗ¬µÄ¹ŁÄÜĶÅŹĒ ”£
£Ø3£©C+E XµÄ»Æѧ·“Ó¦ĄąŠĶŹĒ ·“Ó¦”£
XµÄ»Æѧ·“Ó¦ĄąŠĶŹĒ ·“Ó¦”£
£Ø4£©Š“³öČĪŅāĮ½ÖÖÓėA¾ßÓŠĻąĶ¬¹ŁÄÜĶŵÄAµÄĶ¬·ÖŅģ¹¹ĢåµÄ½į¹¹¼ņŹ½(²»ŗ¬A)£ŗ
Ӣ ӣ
£Ø5£©XÓėĒāŃõ»ÆÄĘČÜŅŗ·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ
ӣ
£Ø6£©ŅŌDĪŖŌĮĻÉś²śŅ»ÖÖ³£¼ūĖÜĮĻµÄ»Æѧ·½³ĢŹ½ŹĒ
ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013½ģ±±¾©µŚ66֊ѧø߶žĻĀѧʌµŚ¶ž“ĪŌĀæ¼»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
£Ø14·Ö£©ĻĀĶ¼ÖŠXŹĒĪŽÖ§Į“µÄ”¢¾ßÓŠ¹ūĻćĪ¶µÄŗĻ³ÉĻćĮĻ£¬æÉÓĆÓŚµ÷Å䶹ÖÖ¹ūĻćŠĶĻć¾«”£ŅŃÖŖDŌŚ±ź×¼×“æöĻĀµÄĆܶČĪŖ1.25 g/L£¬Ęä²śĮææÉŅŌÓĆĄ“ŗāĮæŅ»øö¹ś¼ŅŹÆÓĶ»Æ¹¤·¢Õ¹Ė®Ę½”£EŹĒÉś»īÖŠ³£¼ūµÄŅ»ÖÖÓŠ»śĪļ”£ø÷ĪļÖŹ¼ä×Ŗ»Æ¹ŲĻµČēĻĀ£ŗ

Ēė»Ų“šĻĀĮŠĪŹĢā”£
£Ø1£©A·Ö×ÓµÄĆū³ĘŹĒ ”£
£Ø2£©B·Ö×ÓÖŠĖłŗ¬¹ŁÄÜĶÅŹĒ ”£
£Ø3£©C+E”śXµÄ»Æѧ·“Ó¦ĄąŠĶŹĒ ·“Ó¦”£
£Ø4£©Š“³öČĪŅāĮ½ÖÖÓėA¾ßÓŠĻąĶ¬¹ŁÄÜĶŵÄAµÄĶ¬·ÖŅģ¹¹ĢåµÄ½į¹¹¼ņŹ½£Ø²»ŗ¬A£©£ŗ
Ӣ ӣ
£Ø5£©XÓėĒāŃõ»ÆÄĘČÜŅŗ·“Ó¦µÄ»Æѧ·½³ĢŹ½ ”£
ŅŌDĪŖŌĮĻÉś²śŅ»ÖÖ³£¼ūĖÜĮĻµÄ»Æѧ·½³ĢŹ½ŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010Äź±±¾©ŹŠĪ÷³ĒĒųø߶žĻĀŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌĢā ĢāŠĶ£ŗĢīæÕĢā
(14·Ö) ĻĀĶ¼ÖŠXŹĒĪŽÖ§Į“µÄ”¢¾ßÓŠ¹ūĻćĪ¶µÄŗĻ³ÉĻćĮĻ£¬æÉÓĆÓŚµ÷Å䶹ÖÖ¹ūĻćŠĶĻć¾«”£ŅŃÖŖDŌŚ±ź×¼×“æöĻĀµÄĆܶČĪŖ1.25 g/L£¬Ęä²śĮææÉŅŌÓĆĄ“ŗāĮæŅ»øö¹ś¼ŅŹÆÓĶ»Æ¹¤·¢Õ¹Ė®Ę½”£EŹĒÉś»īÖŠ³£¼ūµÄŅ»ÖÖÓŠ»śĪļ”£ø÷ĪļÖŹ¼ä×Ŗ»Æ¹ŲĻµČēĻĀ£ŗ

Ēė»Ų“šĻĀĮŠĪŹĢā”£
£Ø1£©AµÄĆū³ĘŹĒ ”£
£Ø2£©BÖŠĖłŗ¬µÄ¹ŁÄÜĶÅŹĒ ”£
£Ø3£©C+E XµÄ»Æѧ·“Ó¦ĄąŠĶŹĒ
·“Ó¦”£
XµÄ»Æѧ·“Ó¦ĄąŠĶŹĒ
·“Ó¦”£
£Ø4£©Š“³öČĪŅāĮ½ÖÖÓėA¾ßÓŠĻąĶ¬¹ŁÄÜĶŵÄAµÄĶ¬·ÖŅģ¹¹ĢåµÄ½į¹¹¼ņŹ½ (²»ŗ¬A)£ŗ
Ӣ ӣ
£Ø5£©XÓėĒāŃõ»ÆÄĘČÜŅŗ·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ
ӣ
£Ø6£©ŅŌDĪŖŌĮĻÉś²śŅ»ÖÖ³£¼ūĖÜĮĻµÄ»Æѧ·½³ĢŹ½ŹĒ
ӣ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com