
��ѧ�������֮����ת�������������ʵ��������أ�������������������Ҫ��Ӧ�ã�ͬʱҲ��ѧ���γɻ�ѧѧ����������Ҫ��ɲ��֡�
��1������״̬�£��Ƶĵ��ʺ��Ȼ���������ɿɳ���أ���ͼ9��8����ԭ��ʾ��ͼ����Ӧԭ��Ϊ2Na��FeCl2 Fe��2NaCl���õ�طŵ�ʱ��������ӦʽΪ________________________________________________________________________��
Fe��2NaCl���õ�طŵ�ʱ��������ӦʽΪ________________________________________________________________________��
���ʱ��____________��д�������ƣ��缫�ӵ�Դ�ĸ������õ�صĵ����Ϊ________��
��2��ijͬѧ��ͭƬ��ʯī���缫���һ��Ũ�ȵ�����ͭ��Һ������ԭ��ʾ��ͼ��ͼ��ʾ��һ��ʱ��ֹͣͨ��ȡ���缫�����ڵ������Һ�м���0.98 g������ͭ��ĩǡ����ȫ�ܽ⣬���ⶨ������Һ����ǰ��ȫ��ͬ����ش��������⣺
��Y�缫������________������________�����������ԭ������Ӧ��
�ڵ�������X�缫�Ϸ����ĵ缫��Ӧʽ��_______________________________________________________________________��
�����ڵ������Һ�м���������С�մ�ַ�Ӧ����������ڱ�״������ռ�������__________��
 �Űٷֿ�ʱ����ϵ�д�
�Űٷֿ�ʱ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��ȤС���ͬѧ����ͼ��ʾװ���о��йص绯ѧ������(�ס��ҡ�����������������)�����պϸ�װ�õĿ���Kʱ���۲쵽�����Ƶ�ָ�뷢����ƫת��
��ش��������⣺
(1)�ס��ҡ���������Ϊԭ��ص���__________(��׳ء������ҳء����ء�)��
(2)������F�缫Ϊ__________(�����������������������������������)���óص��ܷ�ӦʽΪ__________��
(3)���ҳ���C�缫��������10.8 gʱ���׳���B�缫����������O2�����Ϊ__________mL(��״��)��
(4)һ��ʱ��Ͽ�����K������������ʹ���ػָ�����ӦǰŨ�ȵ���__________(��ѡ����ĸ)��
| A��Cu | B��CuO | C��CuCO3 | D��Cu2(OH)2CO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���������Ҫ�ɷ�ΪFeTiO3(�ɱ�ʾΪFeO��TiO2)����������MgO��CaO��SiO2�����ʡ������������Ʊ�����ӵ�ص缫����(�����Li4Ti5O12�����������LiFePO4)�Ĺ�ҵ������ͼ��ʾ��
��֪��FeTiO3�����ᷴӦ�����ӷ���ʽΪFeTiO3��4H����4Cl��=Fe2����TiOCl42����2H2O
(1)������FeTiO3����Ԫ�صĻ��ϼ���________��
(2)����A�ijɷ���________��
(3)��ҺB��TiOCl42��ת������TiO2�����ӷ���ʽ��________________��
(4)��Ӧ���й���TiO2ת����(NH4)2Ti5O15��Һʱ��TiԪ�صĽ������뷴Ӧ�¶ȵĹ�ϵ��ͼ��ʾ����Ӧ�¶ȹ���ʱ��TiԪ�ؽ������½���ԭ����________________________________��
(5)��Ӧ�۵Ļ�ѧ����ʽ��________________________��
(6)����ҺD�Ʊ�LiFePO4�Ĺ����У�����17%˫��ˮ��H2C2O4����������________��
(7)�����������(Li4Ti5O12)�����������(LiFePO4)���缫��ɵ�أ��乤��ԭ��ΪLi4Ti5O12��3LiFePO4 Li7Ti5O12��3FePO4���õ�س��ʱ������Ӧʽ��____________________��
Li7Ti5O12��3FePO4���õ�س��ʱ������Ӧʽ��____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����������й�������Һ���ʱ�ı仯�����
(1)��ʯī���缫������ͼװ�õ��AlCl3��Һ���������������ݣ��������г������ɡ�������⣬��������������Һ�л��ɹ۲쵽�������� �����ʹ���������ӷ���ʽ�� ��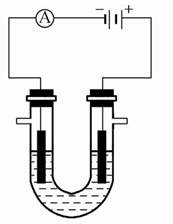
(2)����ʯī���缫���NaCl��Al2(SO4)3�Ļ����Һ�������Һ�ж��ߵ����ʵ���Ũ�ȷֱ�Ϊ3 mol��L-1��0.5 mol��L-1�������б�ʾ�����̵�������ȷ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ͼ��ʾװ�����ӣ�X��Y��Ϊ���Ե缫����ش��������⣺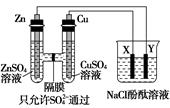
��1��ZnΪ________����
��2�����Ӻ�װ�ú��ձ��е���Һ������Ӧ�����ӷ���ʽ��___________��
��3��ͼ��ͨ����Ĥ��SO42-��________������ҡ�������Ǩ�ƣ�Y�����丽�����ֵ�������________��
��4�������£���Zn����������32.5 gʱ��X����������8.4 L����״����������ʱ�ձ�����Һ�����Ϊ500 mL�����ʱ�ձ�����Һ��pH��________�����������ɵ���������ˮ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1�����ڳ�ʪ�Ŀ������������绯��ʴ�����������(Fe2O3.xH2O)��������ʴʱ�����Ϸ�����Ӧ�ĵ缫��ӦʽΪ__________________��
��2��������Щװ�ÿɷ�ֹ��������ʴ__________________��
��3����ʵ�������У����������ı����϶�ͭ��ֹ������ʴ��װ��ʾ��ͼ������ͼ��
�ٵ��ʱ���Ƽ����Դ��______________�����ӣ�A�缫��Ӧ�Ľ�����______________��дԪ�����ƣ���B�缫�ĵ缫��Ӧʽ��______________________��
������ͼ��һ��������ܵ�صĽṹʾ��ͼ��M��Na2O��Al2O3�Ƶã��������ǵ����
��Ĥ���õ�ط�ӦΪ ���õ�������ĵ缫��ӦʽΪ___________________________��
���õ�������ĵ缫��ӦʽΪ___________________________��
�øõ������Դ��������������ͭʱ������Ƴ������缫��������ʼ��ͬ�������ɺ�ȡ��ϴ������ɡ�����������������Ϊ25.6g���������ϸõ�ظ������ĵ�����Ϊ_________g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������ǿ������������ԭ�ֶΣ��ڻ���������������Ҫ��Ӧ�á���ش��������⣺
(1)��ͭΪ��������ʯīΪ��������NaCl��Һ�����Һ���е�⣬�õ��뵼�����Cu2O��һ�������Դ����������ӦʽΪ__________________��������ӦʽΪ________��
(2)ijͬѧ�����ͼ��ʾ��װ��̽�������ĸ�ʴ����������жϺ�������____________(�����)��
a������ͭƬ�������ݲ���
b��������Ƭ�ĵ缫��ӦʽΪ2Cl����2e��===Cl2��
c�����ȹ۲쵽��ɺ�ɫ�������Ǣ���
d�������͢�����ͭƬ���������������仯
(3)�����о����֣��ø�Ĥ��ⷨ������Ũ����ȩ��ˮ�Ĺ��վ������̼��ܺĽϵ͵��ŵ㣬��ԭ����ʹ��ȩ�ֱ�����������������Ӧ�����Ҵ������ᣬ�ܷ�ӦʽΪ2CH3CHO��H2O CH3CH2OH��CH3COOH
CH3CH2OH��CH3COOH
ʵ�����У���һ��Ũ�ȵ���ȩ?Na2SO4��ҺΪ�������Һ��ģ����ȩ��ˮ�Ĵ������̣���װ����ͼ��ʾ��
�����Լ������ȼ�ϵ��Ϊֱ����Դ����ȼ�ϵ����b��Ӧͨ��__________(�ѧʽ)���缫��ӦʽΪ____________________���������У�������Na2SO4�����ʵ���________(���������С�����䡱)��
����ʵ�ʹ��մ����У���������ȩ��ȥ���ʿɴ�60%�������������ֱ�ע��1 m3��ȩ����Ϊ3 000 mg��L��1�ķ�ˮ���ɵõ��Ҵ�________kg(����������С�����һλ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij����С��ͬѧ����ͼװ�ý���ʵ��,�Իش���������:
(1)����ʼʱ����K��a����,��B���ĵ缫��ӦΪ ����
(2)����ʼʱ����K��b����,��B���ĵ缫��ӦΪ������������,�ܷ�Ӧ�����ӷ���ʽΪ�������������������й�����ʵ��,����˵����ȷ����(�����)��������
����Һ��Na+��A���ƶ����ڴ�A�����ݳ���������ʹʪ��KI������ֽ�������۷�Ӧһ��ʱ������������ɻָ������ǰ����ʵ�Ũ�ȡ�������״����B������2.24 L����,����Һ��ת��0.2 mol����
(3)��С��ͬѧģ�ҵ�������ӽ���Ĥ�����ռ�ķ���,������������ͼװ�õ���������Һ����ȡ������������������������ء�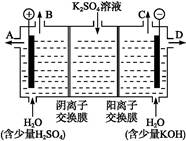
�ٸõ��۵�������ӦΪ����
��ʱͨ�������ӽ���Ĥ��������������������(����ڡ���С�ڡ����ڡ�)ͨ�������ӽ���Ĥ����������
��ͨ�翪ʼ��,����������ҺpH������,�����ԭ����������������������������������
�������Ƶõ�����������������������Һ���Ϊ����ȼ�ϵ��,���������ĵ缫��ӦΪ��������������������������������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���հ�ɽ��������������ɽ���ڣ�������½���ɽһ�������������׳ơ������͡�������������Ҫ�Ŀ���֮һ�������б���Ϊ����ʯ�����������ߣ�����ұ���̸ֵ���Ҫԭ�ϡ�����ʯ��Ҫ�ɷ��д�����Fe3O4��������FeCO3���̿�MnO2��MnCO3��ʯ��Mg3Si3O7(OH)4�ȡ���ҵ�Ͻ�����ʯ����������������Ĥ��ⷨ���¼�����ȡ�����̲��Ƶ���ɫ��Чˮ��������K2FeO4������ҵ�������£�
��1����ҵ��Ϊ���ϡ�����ȡЧ��һ���ȡ�Ĵ�ʩ�ǣ�����д���ַ�����
�� ��
��2��ʯ��ѧʽΪMg3Si3O7(OH)4Ҳ���Ա�ʾ����������ʽ�����������ʽΪ ��
��3����֪��ͬ����������������������������pH���±���
| ���� | Fe3+ | Al3+ | Fe2+ | Mn2+ | Mg2+ |
| ��ʼ������pH | 2.7 | 3.7 | 7.0 | 7.8 | 9.3 |
| ��ȫ������pH | 3.7 | 4.7 | 9.6 | 9.8 | 10.8 |

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com