

���� ��1������ͼ������õ��ʱ䣬��ע���ʾۼ�״̬д���Ȼ�ѧ����ʽ��
��2���������ͷ�Ӧ�Ļ�ܣ��ӿ췴Ӧ���ʣ������ı仯ѧƽ��״̬��
��3���ټ��Ǻ��º�������������ȼ�ϱ仯���㷴Ӧ����������Ϸ�Ӧ���ʸ������õ������ķ�Ӧ���ʣ�
�ڸ���ת���ʵ��ڱ仯���ͳ�ʼ���ı�ֵ�����㣻
�����ݼ�ֵת������õ�ȡֵ��Χ��
��4���������Ǻ��º�ѹ��������Ӧ������ѹǿ�ȼ�������Ӧ������еij̶ȴ�
��� �⣺��1��ͼ�������֪��������������Ϊ��������ķ�Ӧ�����У�ÿ2mol��������Ӧ����198KJ���Ȼ�ѧ����ʽΪ��2SO2��g��+O2��g��?2SO3��g������H=-198 kJ/mol��
�ʴ�Ϊ��2SO2��g��+O2��g��?2SO3��g������H=-198 kJ/mol��
��2����ҵ�ϳ�����V2O5���������������ͷ�Ӧ�Ļ�ܣ��ӿ췴Ӧ���ʣ������ı仯ѧƽ��״̬�����ı䷴Ӧ�ʱ䣬��������b���ϣ�
�ʴ�Ϊ��b��
��3����2SO2��g��+O2��g��$\frac{\underline{����}}{��}$2SO3��g������H=-198 kJ/mol�������ݻ�Ϊ2L���ܱ������г���2mol SO2��g����1mol O2��g������ͼ2����ʾ����2min�ﵽƽ�⣬��÷ų�����Ϊ178.2kJ����Ӧ���������ʵ���=$\frac{178.2kJ}{198kJ}$��1mol=0.9mol��������ʾ�ķ�Ӧ����=$\frac{\frac{0.9mol}{2L}}{2min}$=0.225mol/L•min��
�ʴ�Ϊ��0.225 mol•L-1•min-1��
�ڷ�Ӧ���������ʵ�����0.9mol���������ĵĶ�����������ʵ�����1.8mol��ת������$\frac{1.8mol}{2mol}$��100%=90%���ʴ�Ϊ��90%��
�����ݻ�ѧƽ������ʽ��ʽ����
2SO2��g��+O2��g��$\frac{\underline{����}}{��}$2SO3��g��
��ʼ����mol�� 2 1 0
�仯����mol�� 1.8 0.9 1.8
ƽ������mol��0.2 0.1 1.8
�ٳ���1molO2 0.2 1.1 1.8
��ֵת�� 0 0.9 2
���ٳ���1mol O2���´ﵽƽ��ʱ��ƽ�������ƶ���SO2��ת���ʻ����ӣ����ݼ�ֵת�����������ʵ�������1.8-2֮�䣬SO3ƽ��Ũ�ȵ�ȡֵ��Χ��0.9mol/L��c��SO3����1 mol/L��
�ʴ�Ϊ������0.9mol/L��c��SO3����1 mol/L��
��4������2mol SO2��g����1mol O2 ��g�������ݻ��ɱ���������У���ʼ���Ϊ2L���������Ǻ��º�ѹ��������Ӧ������ѹǿ�ȼ�������Ӧ������еij̶ȴ�
�ﵽƽ��ʱ�ų�����Q kJ��Q��178.2KJ��
�ʴ�Ϊ������
���� ���⿼�����Ȼ�ѧ����ʽ����Ӧ�ú���д����ѧƽ��Ӱ�����ط����жϣ���ѧƽ��ļ���Ӧ�ã���ֵת���dz����ж�����ȡֵ�ķ��������ջ����ǹؼ�����Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ʵ����ʵ | ���� | |
| A | KClO3��SO3����ˮ���ܵ��� | KClO3��SO3Ϊ����� |
| B | SO2ͨ�����ᱵ��Һ���ְ�ɫ���� | BaSO3������ǿ�� |
| C | ��CO2ͨ��NaAlO2��Һ�������ɫ���� | ���H+��������HCO${\;}_{3}^{-}$��AlO${\;}_{2}^{-}$ |
| D | �����°�����ȼ���������ڷŵ�ʱ����������Ӧ | �ǽ����ԣ�P��N |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NO2��SO2��BF3��NCl3������û��һ��������ԭ�ӵ��������Ӷ�������8�����ȶ��ṹ | |
| B�� | ���ʵľ�����һ�������ڵ����������� | |
| C�� | P4��CH4����������������Ҽ��Ƕ�Ϊ109��28�@ | |
| D�� | NaCl��������ÿ��Na+��������������Cl-����12�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
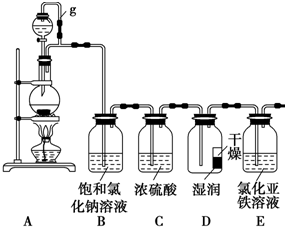 ij̽��С��Ϊ̽�����������ʣ����������ʵ��װ�ã���ش��������⣺
ij̽��С��Ϊ̽�����������ʣ����������ʵ��װ�ã���ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ����£�ȼ��1mol S�ų�������Ϊ297.23 kJ | |
| B�� | S��g��+O2��g���TSO2��g���ų�����������297.23 kJ | |
| C�� | S��g��+O2��g���TSO2��g���ų�������С��297.23 kJ | |
| D�� | �γ�1molSO2�Ļ�ѧ�����ͷŵ����������ڶ���1molS��s����1molO2��g���Ļ�ѧ�������յ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��0.10mol•L-1CH3COONa��Һ��ͨHCl��c��Na+����c��CH3COOH��=c��Cl-�� | |
| B�� | ��0.10mol•L-1NH4HCO3��Һ��ͨCO2��c��NH4+���Tc��HCO3-��+c��CO32-�� | |
| C�� | ��0.10mol•L-1NaHSO3��Һ��ͨNH3��c��Na+����c��NH4+����c��SO32-�� | |
| D�� | ��0.10mol•L-1 Na2SO3��Һ��ͨSO2��c��Na+���T2[c��HSO3-��+c��SO32-��+c��H2SO3��] |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com