
ijͬѧ����ʵ���������Fe��ˮ������Ӧ��ʵ�飬װ����ͼ�ס������֡�
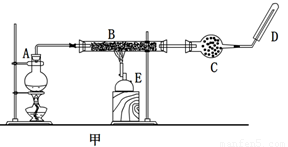
��֪��B�з����������ʯ���Ļ���C�зŵ��Ǹ������EΪ�ƾ���ƣ�GΪ������˿���ֵľ�
���ơ��Ա���װ�ã��ش��������⣺
��1����μ����װ�õ������ԣ� ��
��2����װ����ʪɳ�ӵ������ǣ� ��
��3��B��������Ӧ�Ļ�ѧ����ʽ�� ��
��4����ȡmg����������ʯ����ϣ�Ȼ�������������ų������ռ��������������Ϊ��״��ΪVL�����۵�ת����Ϊ ���г�����ʽ���ɣ����ػ���
��5��Ϊ��֤����Ӧ��Ĺ��������к���+3�۵�Fe����ͬѧȡ��������������Թ��У�����һ����������ʹ���������ܽ⣬���ˣ���������Һ�еμ�KSCN��Һ������۲쵽��Һ����ɫû�仯������˼������ͬѧ��Ϊ��������˵����Ӧ��Ĺ��������в�����+3��Fe�����������ǣ� _��
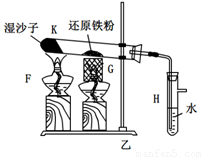
��1����H�м���ˮû�����ܿڣ����Ӻ�װ�ã���K����H�е��ܿڳ������ݣ�ֹͣ���Ⱥ��г���ˮ����֤�����������ã�2�֣�����������Ҳ���֣�
��2���ṩˮ������1�֣� ��3��3Fe��4H2O Fe3O4��4H2
Fe3O4��4H2
��4�� ��
��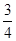 ��56��
��56��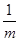 ��100%��1�֣���ע�����������ȷ�Ĵ���ʽ���ɣ���
��100%��1�֣���ע�����������ȷ�Ĵ���ʽ���ɣ���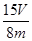 ��100% ��
��100% ��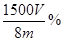 ��
��
��5�����ۿ�����ʣ�࣬ʣ�����ۻ���Fe3+��Ӧ����Fe3+��Һ����ȫ��ԭΪFe2+����1�֣�
��������
�����������1��װ�������Եļ���һ����á�עˮ��������˸�����װ�õ��ص��֪��������װ�õ�ʵ���������H�м���ˮû�����ܿڣ����Ӻ�װ�ã���K����H�е��ܿڳ������ݣ�ֹͣ���Ⱥ��г���ˮ����֤�����������á�
��2�����ٸ�������ˮ������ӦС���ṩ��Ӧ��ˮ����������ʪɳ�ӵ��������ṩˮ������
��3������������ˮ������Ӧ�������������������������B��������Ӧ�Ļ�ѧ����ʽΪ3Fe��4H2O Fe3O4��4H2��
Fe3O4��4H2��
��4����״���������������VL�������������ʵ����� mol�����Ը��ݷ�Ӧʽ3Fe��4H2O
mol�����Ը��ݷ�Ӧʽ3Fe��4H2O Fe3O4��4H2��֪���μӷ�Ӧ���������ʵ�����
Fe3O4��4H2��֪���μӷ�Ӧ���������ʵ����� ��
��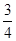 mol����������
mol���������� ��
��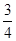 mol��56g/mol���������۵�ת����Ϊ
mol��56g/mol���������۵�ת����Ϊ ��
��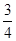 ��56��
��56��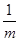 ��100%��
��100%��
��5����Ϊ�ٷ�Ӧ�����ۿ�����ʣ�࣬ʣ�����ۻ���Fe3+��Ӧ������Һ��Fe3+��ȫ��ԭΪFe2+����������Һ�еμ�KSCN��Һ��δ�۲쵽��Һ����ɫû�仯��
���㣺��������ˮ������Ӧԭ����ʵ��̽����ת���ʵ��йؼ����Լ������ӵļ����


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ɽ��ʡ̩�����������и�����һ���¿���ѧ�Ծ����������� ���ͣ�ʵ����
(11��)ijѧ��������6.0 mol/L��H2SO4 1 000 mL��ʵ���������ֲ�ͬŨ�ȵ����
��480 mL 0.5 mol/L �������150 mL 25%������(����1.18 g/mL)����������18 mol/L�����ᡣ�����ֹ�������ƿ��250 mL��500 mL��1 000 mL����ʦҪ��Ѣ٢���������ȫ�����꣬����IJ����ɢ������䡣
��ش��������⣺
(1)ʵ������25%����������ʵ���Ũ��Ϊ______mol/L(����1λС��)��
(2)���Ƹ�������ҺӦѡ������ƿ�Ĺ��Ϊ______mL��
(3)����ʱ����ͬѧ�IJ���˳�����£��뽫��������B��D����������
A�����٢�����Һȫ�����ձ��л�Ͼ��ȣ�
B������Ͳȷ��ȡ�����18 mol/L��Ũ����____mL���ز����������������Һ�С����ò��������裬ʹ���Ͼ��ȣ�
C������Ͼ��ȵ������ز�����ע����ѡ������ƿ�У�
D��_________________________________________________________________
_________________________________________________________________
E��������������ƿ�м�ˮ��ֱ��Һ��ӽ��̶���1��2 cm ����
F�����ý�ͷ�ιܼ�ˮ��ʹ��Һ�İ�Һ��ǡ����̶������У�
G��������ƿ�ǽ�����ҡ�ȡ�
(4)���ʡ�Բ���D����������ҺŨ���к�Ӱ�죿________(�ƫ����ƫС������Ӱ�족)��
(5)���в���Cǰ����ע��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ�긣��ʡ������ѧ�ڵ�һ���¿���ѧ�Ծ��������棩 ���ͣ������
ijѧ��������6��0 mol��L��1��H2SO4 1 000 mL��ʵ���������ֲ�ͬŨ�ȵ������480 mL 0��5 mol��L��1 �������150 mL 25%������(�ѣ�1��18 g��mL��1)����������18 mol��L��1 �����ᡣ�����ֹ�������ƿ��250 mL��500 mL��1 000 mL����ʦҪ��Ѣ٢���������ȫ�����꣬����IJ����ɢ������䡣
��ش��������⣺
��1��ʵ������25%����������ʵ���Ũ��Ϊ________mol��L��1(����1λС��)��
��2�����Ƹ�������ҺӦѡ������ƿ�Ĺ��Ϊ________mL��
��3������ʱ����ͬѧ�IJ���˳�����£��뽫��������B��D����������
A�����٢�����Һȫ�����ձ��л�Ͼ��ȣ�
B������Ͳȷ��ȡ����� 18 mol��L��1��Ũ���� mL���ز����������������Һ�С�
���ò��������裬ʹ���Ͼ��ȣ�
C������Ͼ��ȵ������ز�����ע����ѡ������ƿ�У�
D��______________________________________��
E��������������ƿ�м�ˮ��ֱ��Һ��ӽ��̶���1��2 cm����
F�����ý�ͷ�ιܼ�ˮ��ʹ��Һ�İ�Һ��ǡ����̶������У�
G��������ƿ�ǽ�����ҡ�ȡ�
��4�����ʡ�Բ���D����������ҺŨ���к�Ӱ�죿________(�ƫ����ƫС������Ӱ�족)��
��5�����в���Cǰ����ע��________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ɽ��ʡ̩���и�����һ���¿���ѧ�Ծ��������棩 ���ͣ�ʵ����
ijѧ��������6.0 mol/L��H2SO4 1 000 mL��ʵ���������ֲ�ͬŨ�ȵ����
��480 mL 0.5 mol/L �������150 mL 25%������(�ѣ�1.18 g/mL)����������18 mol/L�����ᡣ�����ֹ�������ƿ��250 mL��500 mL��1 000 mL����ʦҪ��Ѣ٢���������ȫ�����꣬����IJ����ɢ������䡣
��ش��������⣺
(1)ʵ������25%����������ʵ���Ũ��Ϊ______mol/L(����1λС��)��
(2)���Ƹ�������ҺӦѡ������ƿ�Ĺ��Ϊ______mL��
(3)����ʱ����ͬѧ�IJ���˳�����£��뽫��������B��D����������
A�����٢�����Һȫ�����ձ��л�Ͼ��ȣ�
B������Ͳȷ��ȡ�����18 mol/L��Ũ����____mL���ز����������������Һ�С����ò��������裬ʹ���Ͼ��ȣ�
C������Ͼ��ȵ������ز�����ע����ѡ������ƿ�У�
D��_________________________________________________________________
_________________________________________________________________
E��������������ƿ�м�ˮ��ֱ��Һ��ӽ��̶���1��2 cm ����
F�����ý�ͷ�ιܼ�ˮ��ʹ��Һ�İ�Һ��ǡ����̶������У�
G��������ƿ�ǽ�����ҡ�ȡ�
(4)���ʡ�Բ���D����������ҺŨ���к�Ӱ�죿________(�ƫ����ƫС������Ӱ�족)��
(5)���в���Cǰ����ע��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ɽ��ʡ̩���и�����һ���¿���ѧ�Ծ��������棩 ���ͣ�ʵ����
(11��)ijѧ��������6.0 mol/L��H2SO4 1 000 mL��ʵ���������ֲ�ͬŨ�ȵ����
��480 mL 0.5 mol/L �������150 mL 25%������(����1.18 g/mL)����������18 mol/L�����ᡣ�����ֹ�������ƿ��250 mL��500 mL��1 000 mL����ʦҪ��Ѣ٢���������ȫ�����꣬����IJ����ɢ������䡣
��ش��������⣺
(1)ʵ������25%����������ʵ���Ũ��Ϊ______mol/L(����1λС��)��
(2)���Ƹ�������ҺӦѡ������ƿ�Ĺ��Ϊ______mL��
(3)����ʱ����ͬѧ�IJ���˳�����£��뽫��������B��D����������
A�����٢�����Һȫ�����ձ��л�Ͼ��ȣ�
B������Ͳȷ��ȡ�����18 mol/L��Ũ����____mL���ز����������������Һ�С����ò��������裬ʹ���Ͼ��ȣ�
C������Ͼ��ȵ������ز�����ע����ѡ������ƿ�У�
D��_________________________________________________________________
_________________________________________________________________
E��������������ƿ�м�ˮ��ֱ��Һ��ӽ��̶���1��2 cm ����
F�����ý�ͷ�ιܼ�ˮ��ʹ��Һ�İ�Һ��ǡ����̶������У�
G��������ƿ�ǽ�����ҡ�ȡ�
(4)���ʡ�Բ���D����������ҺŨ���к�Ӱ�죿________(�ƫ����ƫС������Ӱ�족)��
(5)���в���Cǰ����ע��
��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com