
 Fe3+ ��2�֣�
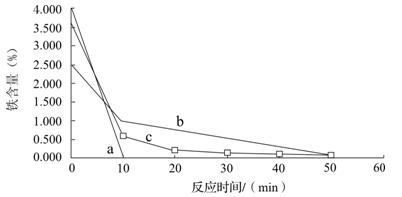
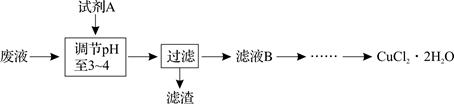
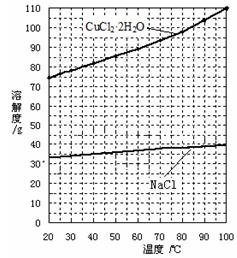
Fe3+ ��2�֣� Cu + FeCl2�����ˣ������ù����м���ϡ�����ܽ������������˳�����ϴ�ӵ�ͭ��(2��)��a (1��) ��ʹ��ϸ���ۣ���ԭ��ϸͭ�ۣ�ͭ�ۿ���ԽС������Ӵ��ı����Խ���ڲ��������ᷴӦԽ�죬������Ч��Խ�ߡ���2�֣�
Cu + FeCl2�����ˣ������ù����м���ϡ�����ܽ������������˳�����ϴ�ӵ�ͭ��(2��)��a (1��) ��ʹ��ϸ���ۣ���ԭ��ϸͭ�ۣ�ͭ�ۿ���ԽС������Ӵ��ı����Խ���ڲ��������ᷴӦԽ�죬������Ч��Խ�ߡ���2�֣�


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 [ʵ���¼]
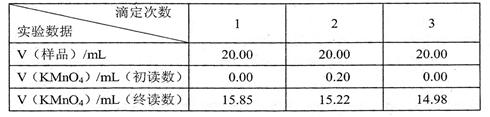
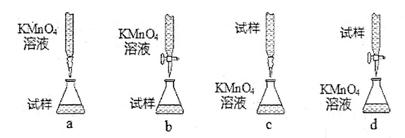
[ʵ���¼] 2����ʵ�����õ�KMnO4����Һ�����ʵ���Ũ��Ϊ
2����ʵ�����õ�KMnO4����Һ�����ʵ���Ũ��Ϊ  ��
��
 ϴһ�£��ټ����ζ����յ㣬������õIJ�Ѫ������Ԫ�صĺ��� ��ƫ��ƫС����Ӱ�죩��
ϴһ�£��ټ����ζ����յ㣬������õIJ�Ѫ������Ԫ�صĺ��� ��ƫ��ƫС����Ӱ�죩���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Cu��Fe3�� | B��Fe2����Fe3�� |
| C��Cu��Cu2����Fe | D��Cu��Fe2����Fe |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
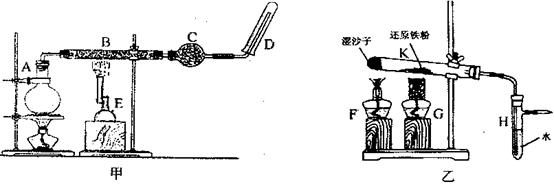
 װ�õļ��쵼�ܴ���ȼ��Ӧ���������壬װ��H�ز����٣�H�������� ��
װ�õļ��쵼�ܴ���ȼ��Ӧ���������壬װ��H�ز����٣�H�������� ��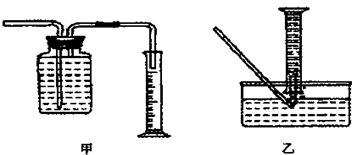
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ͨ������ | B������HCl |
| C������Fe | D������Cu |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��1:3 | B��2:7 | C��2:3 | D��2:1 |
�鿴�𰸺ͽ���>>
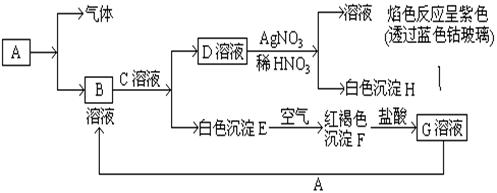
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ɱ�ʾΪ3YO3��3FeO��Fe2O3�� | B���ɱ�ʾΪY3O5��Fe3O4��Fe2O3 |
| C�������ơ����Ļ��ϼ۾�Ϊ��3�� | D�������ơ����Ļ��ϼ۾��У�2����3�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com