
(1)������ˮ��Ʒl�ݣ�ÿ��һ��ʱ��ⶨ����ˮ��Ʒ��pH�������������£�
����ʱ��/h | 0 | 1 | 2 | 3 | 4 |
��ˮ��Ph | 4.73 | 4.62 | 4.56 | 4.55 | 4.55 |
�������ݣ��ش��������⣺
����ˮ��Ʒ��pH�仯��ԭ����(�û�ѧ����ʽ��ʾ)_________________________________��
���������ȡ����������ˮ������ˮ���ϣ�pH����_______��ԭ����_________________(�û�ѧ����ʽ��ʾ) ��
(2)����Ϊ�������������;���ɲ��õĴ�ʩ��_________��
������ú��ȼ�ϡ��ڰѹ����̴���ߡ���ȼ�����������ữ�������м�ʯ�ҡ��ݿ�������Դ
A.�٢ڢ� B.�ڢۢܢ�
C.�٢ۢ� D.�٢ۢܢ�

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���̲���ȫ������ս̰�α�桡���л�ѧ������1 �ս̰�α�� ���ͣ�022
�ҹ�ũҵ������������ÿ����ʧ�ߴ�15�ڶ�Ԫ��Ϊ����Ч�������꣬����Ժ�����ˡ�����������Ͷ���������Ⱦ���������ַ������ȷ��森
(1)������ˮ��Ʒ1�ݣ�ÿ��һ��ʱ��ⶨ����ˮ��Ʒ��pH�������������£�
�������ݣ��ش��������⣺
����ˮ��Ʒ��pH�仯��ԭ��________��(�û�ѧ����ʽ��ʾ)
���������ȡ����������ˮ������ˮ���ϣ�pH����________��ԭ����________��(�û�ѧ����ʽ��ʾ)
(2)����Ϊ�������������;���ɲ��õĴ�ʩ��(����)��
������ú��ȼ�ϡ��ڰѹ����̴���ߡ���ȼ�����������ữ�������м�ʯ�ҡ��ݿ�������Դ
A���٢ڢ�
B���ڢۢܢ�
C���٢ۢ�
D���٢ۢܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������������ ���ͣ�058
�ҹ�ũҵ������������ÿ����ʧ�ߴ�15�ڶ�Ԫ��Ϊ����Ч�������꣬����Ժ�����ˡ�����������Ͷ���������Ⱦ���������ַ������ȷ��森
(1)������ˮ��Ʒ1�ݣ�ÿ��һ��ʱ��ⶨ����ˮ��Ʒ��pH�������������£�

�������ݣ��ش��������⣺
����ˮ��Ʒ��pH�仯��ԭ��(�û�ѧ����ʽ��ʾ)_____________��
���������ȡ����������ˮ������ˮ���ϣ�pH����_________��ԭ����(�û�ѧ����ʽ��ʾ)______________��
(2)����Ϊ�������������;���ɲ��õĴ�ʩ��
[����]
������ú��ȼ�ϡ��ڰѹ����̴���ߡ���ȼ�����������ữ�������м�ʯ�ҡ��ݿ�������Դ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�022
�ҹ�ũҵ������������ÿ����ʧ�ߴ�15�ڶ�Ԫ��Ϊ����Ч�������꣬����Ժ�����ˡ�����������Ͷ���������Ⱦ���������ַ������ȷ��森
(1)������ˮ��Ʒ1�ݣ�ÿ��һ��ʱ��ⶨ����ˮ��Ʒ��pH�������������£�
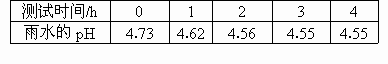
�������ݣ��ش��������⣺
����ˮ��Ʒ��pH�仯��ԭ��(�û�ѧ����ʽ��ʾ)_____________��
���������ȡ����������ˮ������ˮ���ϣ�pH����_________��ԭ����(�û�ѧ����ʽ��ʾ)______________��
(2)����Ϊ�������������;���ɲ��õĴ�ʩ��
[����]
������ú��ȼ�ϡ��ڰѹ����̴���ߡ���ȼ�����������ữ�������м�ʯ�ҡ��ݿ�������Դ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com