
(10��)ijѧ��Ϊ�ⶨδ֪Ũ�ȵ�������Һ��ʵ�����£���1.00mL������������100 mLϡH2SO4��Һ����0.14 mol��L��1��NaOH��Һ�ζ�����ϡ H2SO425mL���ζ���ֹʱ����NaOH��Һ15mL��
��1����ѧ���ñ�0.14 mol��L��1NaOH��Һ�ζ������ʵ��������£�
| A������ʽ�ζ���ȡϡH2SO4 25 mL��ע����ƿ�У�����ָʾ���� | |
| B���ô��ⶨ����Һ��ϴ��ʽ�ζ��ܡ� | C��������ˮϴ�ɾ��ζ��ܡ� |
| D��ȡ�¼�ʽ�ζ����ñ���NaOH��Һ��ϴ����Һע���ʽ�ζ��̶ܿȡ�0������2��3 cm�����ٰѼ�ʽ�ζ��̶ܹ��ã�����Һ�����̶ȡ�0����0���̶����¡� |
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
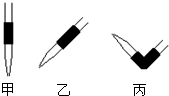 ʵ��������һδ֪Ũ�ȵ�ϡ���ᣬijѧ��Ϊ�ⶨ�����Ũ����ʵ�����н�������ʵ�飮�������������գ�
ʵ��������һδ֪Ũ�ȵ�ϡ���ᣬijѧ��Ϊ�ⶨ�����Ũ����ʵ�����н�������ʵ�飮�������������գ�| ʵ���� | NaOH��Һ��Ũ�� ��mol/L�� |
�ζ����ʱ������NaOH��Һ�������mL�� | ����������Һ�������mL�� |
| 1 | 0.10 | 20.02 | 20.00 |
| 2 | 0.10 | 20.00 | 20.00 |
| 3 | 0.10 | 19.00 | 20.00 |
| 4 | 0.10 | 19.98 | 20.00 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ʵ��������һδ֪Ũ�ȵ�ϡ���ᣬijѧ��Ϊ�ⶨ�����Ũ����ʵ�����н�������ʵ�飺
ʵ��������һδ֪Ũ�ȵ�ϡ���ᣬijѧ��Ϊ�ⶨ�����Ũ����ʵ�����н�������ʵ�飺| ʵ���� | NaOH��Һ��Ũ�� ��mol/L�� |
�ζ����ʱ��NaOH��Һ����������mL�� | ����������Һ����� ��mL�� |
| 1 | 0.10 | 22.62 | 20.00 |
| 2 | 0.10 | 22.72 | 20.00 |
| 3 | 0.10 | 22.80 | 20.00 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(10��)ijѧ��Ϊ�ⶨδ֪Ũ�ȵ�������Һ��ʵ�����£���1.00mL������������100 mLϡH2SO4��Һ����0.14mol��L��1��NaOH��Һ�ζ�����ϡ H2SO425mL���ζ���ֹʱ����NaOH��Һ15mL��
��1����ѧ���ñ�0.14 mol��L��1NaOH��Һ�ζ������ʵ��������£�
A������ʽ�ζ���ȡϡH2SO4 25 mL��ע����ƿ�У�����ָʾ����
B���ô��ⶨ����Һ��ϴ��ʽ�ζ��ܡ� C��������ˮϴ�ɾ��ζ��ܡ�
D��ȡ�¼�ʽ�ζ����ñ���NaOH��Һ��ϴ����Һע���ʽ�ζ��̶ܿȡ�0������2��3 cm�����ٰѼ�ʽ�ζ��̶ܹ��ã�����Һ�����̶ȡ�0����0���̶����¡�
E�����ζ����Ƿ�©ˮ�� F����ȡ��ƿ�����ظ�����һ�Ρ�
G������ƿ���ڵζ������棬ƿ�µ�һ�Ű�ֽ���ߵα�ҡ����ƿֱ���ζ��յ㣬���µζ���Һ�����ڿ̶ȡ�
�ٵζ���������ȷ˳����(�������д)�� ����������������������������������
�ڸõζ�������Ӧѡ�õ�ָʾ���ǣ� ����������
����G���������ȷ���յ�? ������
��
��2����ʽ�ζ���������ˮ��ϴ��δ�ñ�Һ��ϴ���µζ����(�ƫС������ƫ��ǡ�ú��ʡ�) ��ԭ���� ��
��3�������������(ϡ��ǰ������)��Һ�����ʵ���Ũ��Ϊ ��mol��L��1 (����������С������λ)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��㶫ʡ½������ʯ��ѧ�߶���ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ������
ʵ��������һδ֪Ũ�ȵ�ϡ���ᣬijѧ��Ϊ�ⶨ�����Ũ����ʵ�����н�������
ʵ�顣�������������գ�
����һ������100mL 0.10mol/L NaOH����Һ��.
�������ȡ20.00mL����ϡ���������ƿ�У����μ�2��3�η�̪��Һ��ָʾ�������Լ����Ƶı�NaOH��Һ���еζ����ظ������ζ�����2��3�Σ���¼�������¡�
| ʵ���� | NaOH��Һ��Ũ�� ��mol/L�� | �ζ����ʱ������NaOH��Һ�������mL�� | ����������Һ�������mL�� |
| 1 | 0.10 | 22.02 | 20.00 |
| 2 | 0.10 | 22.00 | 20.00 |
| 3 | 0.10 | 21.98 | 20.00 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015���Ĵ�ʡ�߶���ѧ�ڵ������¿���ѧ�Ծ��������棩 ���ͣ������
��. ��ˮ��Һ�гȺ�ɫ��Cr2O72-���ɫ��CrO42-������ƽ���ϵ��
Cr2O72-��H2O  2CrO42-��2H+����K2Cr2O7����ˮ���ϡ��Һ�dz�ɫ��
2CrO42-��2H+����K2Cr2O7����ˮ���ϡ��Һ�dz�ɫ��
��1����������Һ�м���NaOH��Һ����Һ�� ɫ����Ϊ ��
��2�����Ѽ���NaOH��Һ�ģ�1�����ټ������ϡH2SO4������Һ�� ɫ����Ϊ ��
��3����ԭ��Һ�м���Ba(NO3)2��Һ����֪BaCrO4Ϊ��ɫ��������ƽ���� �����ƶ�����Һ��ɫ�� ��������������dz�����䡱��
��.ʵ������һδ֪Ũ�ȵ�ϡ���ᣬijѧ��Ϊ�ⶨ�����Ũ����ʵ�����н�������ʵ�飺
1.����100mL 0.10mol/L NaOH����Һ��
2.ȡ20.00mL����ϡ������Һ������ƿ�У����μ�2��3�η�̪��ָʾ�������Լ����Ƶı�NaOH��Һ���еζ���
3.�ظ������ζ�����2��3�Σ���¼�������¡�
|
ʵ���� |
NaOH��Һ��Ũ�� ��mol/L�� |
�ζ����ʱ��NaOH��Һ����������mL�� |
����������Һ����� ��mL�� |
|
1 |
0.10 |
22.62 |
20.00 |
|
2 |
0.10 |
22.58 |
20.00 |
|
3 |
0.10 |
22.60 |
20.00 |
��1���ζ��ﵽ�յ�������� ����ʱ��ƿ����Һ��pH��ΧΪ ��
��2�������������ݣ��ɼ�����������Ũ��ԼΪ ��
��3����ȥ��ʽ�ζ��������ݵķ���Ӧ������ͼ �IJ�����Ȼ��ѹ������ʹ���첿�ֳ�����Һ��



�� �� ��
��4��������ʵ���У����в���������������ȷ������ɲⶨ���ƫ�ߵ��� ����ѡ�۷֣���
A���ζ��յ����ʱ���Ӷ���
B����ʽ�ζ���ʹ��ǰ��ˮϴ��δ�ô���������Һ��ϴ
C����ƿˮϴ��δ����
D������NaOH����Һʱ��û�е��ܽ�Һ�������¾�ת��������ƿ��
E������NaOH����Һʱ������ʱ��������ƿ�Ŀ̶���
F����ʽ�ζ��ܼ��첿�������ݣ��ζ�����ʧ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com