
A��J����ѧ��ѧ�г��������ʣ�����֮���ת����ϵ���¿�ͼ��ʾ�����ֲ�������ȥ������֪A��һ�ָ��۵����ʣ�J��һ�ֺ��ɫ������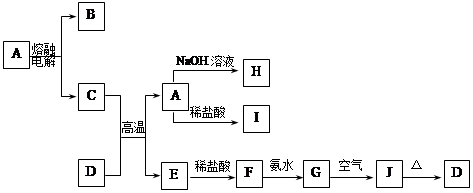
��ش��������⣺
��1��A�Ļ�ѧʽΪ ����ˮ�е��ܽ��� ��ѡ����ܡ��������ܡ��������ܡ�����
��2��H��Һ��ͨ�������CO2���䷴Ӧ�����ӷ���ʽ�� ��
G��J�Ļ�ѧ����ʽΪ ��
��Ӧ�������� ��
��3��D����ǡ������һ������ϡ������ú��ʵĻ�ѧ�����ʾ������Һ�����Ե�ԭ�� ��
��1��Al2O3 ������
��2��AlO2-+CO2+H2O�TAl(OH)3��+HCO3-,4Fe(OH)2+2H2O+O2�T4Fe(OH)3��
��3��Fe3++3H2O?Fe(OH)3+3H+
���������������1��A��һ�ָ��۵����ʣ����ڸ����µ�⣬ӦΪ����������BΪO2��D��һ�ֺ���ɫ���壬ӦΪFe2O3����Al�������ȷ�Ӧ����EΪFe�����ݷ�Ӧ��ϵ��֪HΪNaAlO2��IΪAlCl3��FΪFeCl2��GΪFe(OH)2��JΪFe(OH)3����A�Ļ�ѧʽΪAl2O3 ��ˮ�е��ܽ���Ϊ���ܡ�
��2������������֪HΪNaAlO2��GFe(OH)2��Ϊ��H��Һ��ͨ�������CO2�����ӷ���ʽ��AlO2-+CO2+H2O�TAl(OH)3��+HCO3-��G��J�Ļ�ѧ����ʽΪΪ4Fe(OH)2+2H2O+O2�T4Fe(OH)3����Ӧ����Ϊ�ɰ�ɫ��Ϊ����ɫ�����ձ�Ϊ���ɫ������
��3��D����ǡ������һ������ϡ����������Ȼ�����Һ����ˮ����Һ�����ԣ���Fe3++3H2O?Fe(OH)3+3H+���ʴ�Ϊ��Fe3++3H2O?Fe(OH)3+3H+��
���㣺Ԫ�ؼ�����������ʼ��ƶϡ�����ˮ��


 �����������һ��һ��ϵ�д�
�����������һ��һ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ʶͼ��ͼ����Ҫ�ļ��ܡ���Ҫ��ش��������⣺
��1����̼������Һ�У���μ������ᣬ������������ʵ������������������ͼ��ʾ����OA�η�����Ӧ�����ӷ���ʽΪ________��AB�η�����Ӧ�����ӷ���ʽΪ________��
��2������ͼ�в��������ʯ��ˮ��ͨ��CO2�����ɳ���������CO2����Ĺ�ϵͼ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��10.1�˹������ƺ��ƵĻ�����120.1��ˮ��ַ�Ӧ���ռ�������������ڱ�״����Ϊ2.24L��
��1���Էֱ�д���������Ƹ�ˮ��Ӧ�����ӡ���ѧ����ʽ ����2������ԭ������й������ƺ������Ƶ����ʵ���֮�ȣ�n(Na2O2)��n(Na)�� ��
��3������������Һ�����������Ƕ�أ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����ʵķ�����ᴿ�ж��ַ��������ʷ��롢�ᴿ����������ڿ�ѧ�о���ҵ������ռ��ʮ����Ҫ�ĵ�λ����ҵ��ұ������ԭ����������(��Ҫ�ɷ���Al2O3������ΪFe2O3��SiO2�ȣ���֪SiO2�Dz�����ˮ�����������Fe2O3�Dz�����ˮ�ļ���������)��ij�о�С����Ƶ��ᴿAl2O3�ķ������£�
��1��д������A�Ļ�ѧʽ��___________________________________________________��
��2�����������NaOH��Һ�����˺����Һ�к��е�������________________________��
��3��д���ɳ���A����Al2O3�Ļ�ѧ����ʽ��________________________��ͨ�����CO2���ɳ���Aʱ��Ӧ�����ӷ���ʽΪ_______________________________________________��
����Ҫ��ش��������⣺
A��B��C��D��E�dz�����������ʣ�������ת����ϵ (��ȥ��������Ʒ����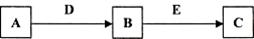
��1����AΪ����ɫ���嵥�ʣ�D��EΪ�����г��������ֽ���������E��һ�ֺ�ɫ���������ʡ�
�� д����B����Һ�м�������D�����ӷ�Ӧ����ʽ_____________________________________��
��������õ���Һ�м���NaOH��Һ�����ڿ����з��õ������ǣ�_________________________��д�������ڿ����з��õĻ�ѧ��Ӧ����ʽ��__________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������ת����ϵ�Լ�����ش�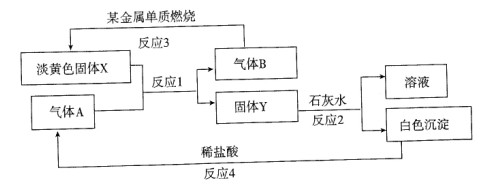
��1������X�������� ��Y�Ļ�ѧʽ�� ��
��2��д����Ӧl�Ļ�ѧ����ʽ ��
��3��д����Ӧ2�Ļ�ѧ����ʽ ��
��4����15��6g X������ˮ��Ӧ��ת�� mol e-��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(15��)�ռ�(NaOH)�ʹ���(Na2CO3)�����ᡢ��������Ტ��Ϊ��ҵ�ϵġ������������Ҫ��ش��������⣺
��1����������й�NaOH��Һ�μӷ�Ӧ�����ӷ���ʽ
����NaOH��Һ�м�����Ƭ��_______________________��
����NaOH��Һ�еμ������Ȼ�����Һ___________________��
�۶���������NaOH��Һ����������ԭ��Ӧ��___________________��
�����ȵ�NaOH��Һϴ��մ�����ʵ��Թܣ�___________________��
��2��������̼���ƺ�̼�����Ƶ����ʱȽϣ��á�������������������գ�
�����ȶ��ԣ�Na2CO3_______NaHCO3��
����ϡ���ᷴӦ������Na2CO3_______NaHCO3��
�����ʵ�����ͬʱ��������������ʵ�����Na2CO3_______NaHCO3��
��3����10 mL 1 mol/L�Ĵ�����Һ�У����Ͻ��貢��μ���10 mL 1.5 mol/L���ᣬ��ȫ��Ӧ���ڱ�״�������ɶ�����̼�����Ϊ____________L��
��4��̼�����ڹ�ҵ�Ͼ��й㷺�����ã�̼���ƿ����������й�ҵ������Ҫԭ�ϵ���_____(����)
a������ b��ˮ�� c����ֽ d����ˮ��ȡþ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ʵ�����ijͬѧȡһС�����������ˮ��Ӧ��ʵ�顣������������⣺
�п��Ľ����Ʊ�¶�ڿ����У����ȹ۲쵽�������� ����������Ӧ�Ļ�ѧ����ʽ�� ��
(2)����Ͷ��ˮ�к����ڻ���һ��С������һ�������ܵó��Ľ����ǣ�
�� ���� ��
��һС����Ͷ��ʢ�б���ʯ��ˮ���ձ��У������ܹ۲쵽�������� �����ţ���
| A������������ | B�����ڻ���С����Һ�����ζ� |
| C����Һ�ײ�������ɫ�Ľ��������� | D����Һ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ��������Ĺ�������ʾ��ͼ���£�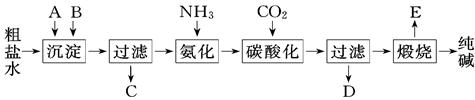
���������գ�
��1������ˮ���������A��B������(������A��Դ��ʯ��Ҥ��)��д��A��B�Ļ�ѧʽA�� ��B.
��2��ʵ�����ᴿ���ε�ʵ���������Ϊ��ȡ���� �������� �� ����ȴ�ᾧ�� �����
��3����ҵ��������������У�̼�ữʱ������������ ��̼�ữʱû������̼���ƾ��壬��ԭ����
��4��̼�ữ����ˡ���ҺD����Ҫ�ijɷ��� (��д��ѧʽ)��������һ�ɷֵ������ӵľ��巽����
(5)��������а���ѭ��ʹ�õģ�Ϊ�ˣ���ҺD����ʯ��ˮ����������ʯ��ˮ���������ķ�Ӧ�����ӷ�ʽ��Ϊ ����ҺD��ʯ��ˮǰ��Ҫ���ȣ�ԭ����
(6)��Ʒ�����к���̼�����ƣ�����ü��ȷֽ�ķ����ⶨ������̼�����Ƶ�����������������̼�����Ƶ����������ɱ�ʾΪ (ע����ı���ʽ�����õ��йط��ŵĺ���)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��1���֣�
һλͬѧ�ڸ�ϰʱ��������һ��ϰ�⣺ij��ɫ��Һ�п��ܺ��С�H+��OH-��Na+��NO3-�����������ۺ�ֻ����H2���ʸ���ɫ��Һ���ܴ��������ļ������ӡ�
��1���������۲���H2��˵��������______��������ԡ���ԭ�ԡ�����
��2����ͬѧ��������H+�������ڣ���NO3-�Ͳ��ܴ������ڡ�
���ʵ��֤ʵ���£�
| װ �� | �� �� |
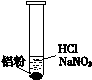 | ��. ʵ���ʼ��δ���������� ��. ��һ������������ݣ�Һ���Ϸ���dz��ɫ ��. �Թܱ��ȣ���Һ���� |
| ʵ �� | �� �� | �� �� |
| ʵ��1 | ��ʪ��KI��������ֽ���ڿ����� | δ���� |
| ʵ��2 | ��ʪ��KI��������ֽ����dz��ɫ���� | ��ֽ���� |
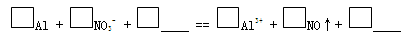
| װ �� | �� �� |
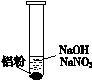 | ��. ʵ���ʼ��δ���������� ��. ��һ������������ݣ��д̼�����ζ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com