
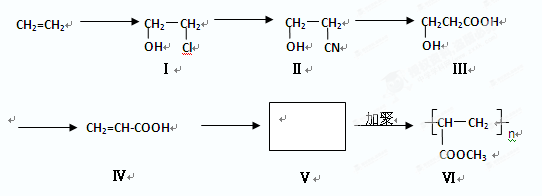
| A��������III���Է���������Ӧ |
| B��������III��������NaOH ��Һ��Ӧ |
| C��������IV�������������ӳɷ�Ӧ |
| D��������III��IV����������Ʒ�Ӧ�������� |
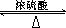 CH2=CH-COOCH3+H2O ��2�֣�
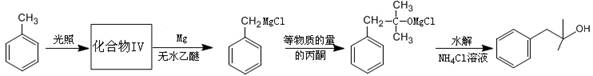
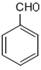
CH2=CH-COOCH3+H2O ��2�֣�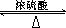 CH2=CH-COOCH3+H2O����5��III�Ĺ��������ǻ����Ȼ������д�����������ʣ����Է���������Ӧ�����������ǿ��������Һ������Ҳ��ȼ����������A��ȷ��III�����Ȼ������Թ����ţ��������ᣬ������NaOH�����кͷ�Ӧ����B����IV�Ĺ�������̼̼˫�����Ȼ�����һ�������£�̼̼˫���������ܷ����ӳɷ�Ӧ����C��ȷ��III��������ܷ����û���Ӧ�����ǻ����Ȼ�����ԭ�ӱ����û�������������IV�������Ҳ�ܷ����û���Ӧ�����Ȼ�����ԭ�ӱ����û���������������D��ȷ�������£�IV��̼̼˫�����巢���ӳɷ�Ӧ��V��̼̼˫�����巢���ӳɷ�Ӧ����˾���ʹ������Ȼ�̼��Һ��ɫ����E��ȷ����6��I�Ĺ��������ǻ�����ԭ�ӣ����д���±���������ʣ���Cu���£������Ǽ�����������Ϊȩ������2HOCH2CH2Cl+O2
CH2=CH-COOCH3+H2O����5��III�Ĺ��������ǻ����Ȼ������д�����������ʣ����Է���������Ӧ�����������ǿ��������Һ������Ҳ��ȼ����������A��ȷ��III�����Ȼ������Թ����ţ��������ᣬ������NaOH�����кͷ�Ӧ����B����IV�Ĺ�������̼̼˫�����Ȼ�����һ�������£�̼̼˫���������ܷ����ӳɷ�Ӧ����C��ȷ��III��������ܷ����û���Ӧ�����ǻ����Ȼ�����ԭ�ӱ����û�������������IV�������Ҳ�ܷ����û���Ӧ�����Ȼ�����ԭ�ӱ����û���������������D��ȷ�������£�IV��̼̼˫�����巢���ӳɷ�Ӧ��V��̼̼˫�����巢���ӳɷ�Ӧ����˾���ʹ������Ȼ�̼��Һ��ɫ����E��ȷ����6��I�Ĺ��������ǻ�����ԭ�ӣ����д���±���������ʣ���Cu���£������Ǽ�����������Ϊȩ������2HOCH2CH2Cl+O2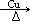 2OHCCH2Cl+2H2O����I�Ĵ���������ΪH2O��CH2ClCHO��
2OHCCH2Cl+2H2O����I�Ĵ���������ΪH2O��CH2ClCHO��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
 ������Ҫ�Ļ���ԭ�ϣ��㷺����������ҵ��
������Ҫ�Ļ���ԭ�ϣ��㷺����������ҵ��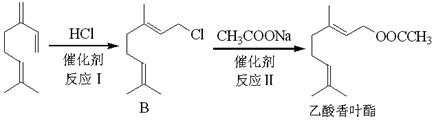

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
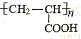 �ĵ��壬��A�����������ŵ������� ��
�ĵ��壬��A�����������ŵ������� ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ƶ���
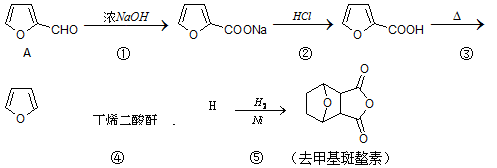
 (Diels-Alder ��Ӧ)
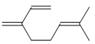
(Diels-Alder ��Ӧ) �Ʊ���ϩ������
�Ʊ���ϩ������ �ĺϳ�·������ͼ�����Լ���ѡ�����ϳ�·������ͼʾ�����£�
�ĺϳ�·������ͼ�����Լ���ѡ�����ϳ�·������ͼʾ�����£�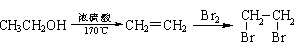
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ƶ���

 Ҳ�ܷ�������V��VI�ķ�Ӧ����д�����ɴ��Ľṹ��ʽ ��
Ҳ�ܷ�������V��VI�ķ�Ӧ����д�����ɴ��Ľṹ��ʽ ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ƶ���
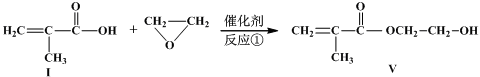
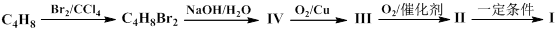
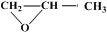 �������Ʒ�Ӧ�ٵķ�Ӧ����д������һ�ֲ���Ľṹ��ʽ ��
�������Ʒ�Ӧ�ٵķ�Ӧ����д������һ�ֲ���Ľṹ��ʽ ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ƶ���
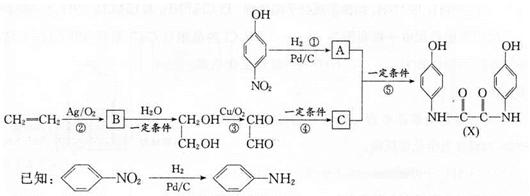
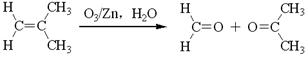
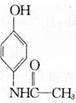 ��ͬ���칹��Ľṹ��ʽΪ______(д����)��
��ͬ���칹��Ľṹ��ʽΪ______(д����)�� �ķ������£�
�ķ������£�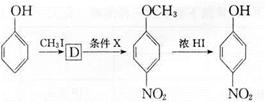
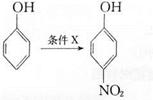
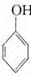 ��
�� Ϊԭ�ϣ����Լ���ѡ�������ٲ���ϳ�
Ϊԭ�ϣ����Լ���ѡ�������ٲ���ϳ�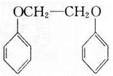 ��д���ϳ�·��(��ʾ�����緽��һ��: ____________��
��д���ϳ�·��(��ʾ�����緽��һ��: ____________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ƶ���
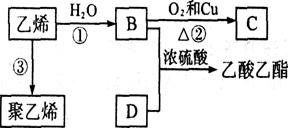
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

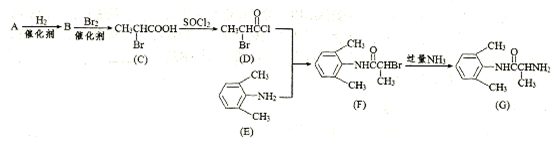
 B�ķ�Ӧ����Ϊ ��
B�ķ�Ӧ����Ϊ ��  E�ķ�Ӧ�У�����Ļ�����X������Cu(OH)2��Ӧ����ש��ɫ�����Ļ�ѧ����ʽΪ
E�ķ�Ӧ�У�����Ļ�����X������Cu(OH)2��Ӧ����ש��ɫ�����Ļ�ѧ����ʽΪ JΪȡ����Ӧ������һ��������еĹ������� ��
JΪȡ����Ӧ������һ��������еĹ������� �� R(C8H7O2Cl)
R(C8H7O2Cl)  S
S T��T�ĺ˴Ź�������ֻ������壬Q�Ľṹ��ʽΪ ��R
T��T�ĺ˴Ź�������ֻ������壬Q�Ľṹ��ʽΪ ��R S�Ļ�ѧ����ʽΪ ��
S�Ļ�ѧ����ʽΪ ��

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com