
A��B��D��E��FΪ������Ԫ�أ��ǽ���Ԫ��A����������������������ͬ��B����������������������������2����B��D�г��ȼ������������ۻ�����BD2��E����D2��������ͬ�ĵ�������A��F��ȼ�գ���������ˮ�õ�һ��ǿ�ᡣ�ش��������⣺
(3)����ЩԪ����ɵ����ʣ�����ɺͽṹ��Ϣ���±���
| ���� | ��ɺͽṹ��Ϣ |
| a | ����A�Ķ�Ԫ���ӻ����� |
| b | ���зǼ��Թ��ۼ��Ķ�Ԫ���ӻ������ԭ����֮��Ϊ1��1 |
| c | ��ѧ���ΪBDF2 |
| d | ֻ����һ�������������ҿɵ���ĵ��ʾ��� |
a�Ļ�ѧʽΪ____________��b�Ļ�ѧʽΪ______________��c�ĵ���ʽΪ________��d�ľ���������______��
(4)��A��B��DԪ����ɵ����ֶ�Ԫ�������γ�һ������Դ���ʡ�һ�ֻ��������ͨ��________�����ɾ��п�ǻ�Ĺ��壻��һ�ֻ�����(��������Ҫ�ɷ�)���ӽ���ÿ�ǻ������ӵĿռ�ṹΪ________��
�𰸡�(3)NaH��Na2O2��Na2C2�� 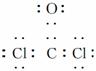 ����������
����������
(4)�⡡��������
������������Ŀ�и����ĸ���Ԫ�صĽṹ�ص�����ʣ��ƶϳ�����Ԫ�أ������⡣����������������������ͬ�Ķ����ڷǽ���Ԫ��ֻ����Ԫ�أ�AΪ��Ԫ�ء�������������������������2���Ķ�����Ԫ�ؿ���Ϊ̼Ԫ�ػ���Ԫ�أ�����B��D�г��ȼ�����ɵ���ۻ�����Ļ�ѧʽΪBD2�ж�BΪ̼Ԫ�أ�DΪ��Ԫ�ء�E����O2��������ͬ�ĵ���������EΪ��Ԫ�ء�H2��Cl2��ȼ�����ɵ�HCl����ˮ�õ�������Ϊǿ�ᣬFΪ��Ԫ�ء�
(3)��H��C��O��Na��Cl��ɵĻ������У�������Ԫ�صĶ�Ԫ���ӻ�����ӦΪNaH����aΪNaH�����зǼ��Թ��ۼ��Ķ�Ԫ���ӻ�����ΪNa2O2��Na2C2����bΪNa2O2��Na2C2��cΪCOCl2��Ϊ���ۻ������ԭ�ӳɼ������8e���ȶ��ṹ������ʽΪ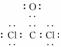 ��ʯī�ͽ����ƾ��ɵ��磬��ʯī�д��ڹ��ۼ������Ӽ��������������͵�����������������ֻ���ڽ����������ڽ������壬��dΪNa��
��ʯī�ͽ����ƾ��ɵ��磬��ʯī�д��ڹ��ۼ������Ӽ��������������͵�����������������ֻ���ڽ����������ڽ������壬��dΪNa��
(4)��H2O��CH4�γɵ�һ������ԴΪ��ȼ����ˮ����֮���ͨ��������ɾ��п�ǻ�Ĺ��壻CH4����������Ҫ�ɷ֣�����ӵĿռ�ṹΪ�������塣



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����������һ�ֳ����Ļ���ԭ�ϡ���SO2ͨ�밴һ��������ɵ�Na2S��Na2CO3�Ļ����Һ�У���ɵõ�Na2S2O3�����Ʊ���Ӧ����ʽΪ2Na2S��Na2CO3��4SO2===3Na2S2O3��CO2��
(1)�����ƻ����Һǰ�Ƚ�����ˮ�������һ��ʱ�����ã���Ŀ����____________��
(2)�ø÷�����õ�Na2S2O3·H2O�����г�����һ���������ʡ�ij��ȤС�����������������ʳɷֽ���̽��(�����Ǹ���Ӧ�����������Ľᾧˮ)��
��������衿������1��������ֻ��Na2CO3����
����2��������ֻ��Na2S����
����3��________________________________________________________________________��
���������ϡ���Na2S2O3�����ԡ�������Һ�н��ȶ�������������Һ����Ѹ�ٷ�Ӧ��Na2S2O3��H2SO4===Na2SO4��S����SO2����H2O��
���ж���˼������ijͬѧȡ�����Ƶõľ�����������ϡH2SO4����������������ͨ��CuSO4��Һ�У�δ����ɫ�������ݴ���Ϊ����2������������Ϊ������Ƿ������________(���������������)��˵�����ɣ�______________________________��
����Ʒ�������ʵ�顿�����ڼ���1������±�ʵ�鷽����������(������ѡ)��
��ѡʵ���Լ���3 mol·L��1H2SO4��1 mol·L��1NaOH������KMnO4��Һ������NaHCO3��Һ��Ʒ����Һ������ʯ��ˮ
| ʵ�鷽�� | ������ |
(3)��֪��2Na2S2O3��I2===2NaI��Na2S4O6��Ϊ�ⶨ���Ƶþ���Ĵ��ȣ���С���Ե�����ָʾ������0.010 mol·L��1�ĵ�ˮ���ж��ȡ���ζ������Na2S2O3·5H2O�ĺ���ԼΪ102%���������Լ��������������������ý������ܵ�ԭ����________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и������ʵľ����У���ѧ��������ͬ����������Ҳ��ͬ����(����)
A��SO3��SiO2 B��CO2��H2O
C��NaCl��HCl D��CCl4��KCl
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й����У����ۼ����ƻ�����(����)
A��������
B������������ľ̿����
C���ƾ�����ˮ
D��HCl��������ˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪����Ԫ�ص�ԭ�������Ĵ�С˳��ΪC��A��B��D��E��A��Cͬ���ڣ�B��Cͬ���塣A��B�γɵ����ӻ�����A2B���������ӵĵ�������ͬ�����������Ϊ30��D��E���γ�4��10�����ӵķ��ӡ��Իش��������⣺
(1)д������Ԫ�ص�Ԫ�ط��ţ�A��________��B��________��C��________��D��________��E��________��
(2)�õ���ʽ��ʾ���ӻ�����A2B���γɹ��̣�__________________________________��
(3)д���������ʵĵ���ʽ��
��DԪ���γɵĵ��ʣ�___________________________________________________��
��E��B�γɵĻ����_________________________________________________��
��A��B��E�γɵĻ����_____________________________________________________��
��D��E�γɵĻ����_____________________________________________��
��C��D��E�γɵ����ӻ����_____________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪һ�������ºϳɰ���Ӧ��N2(g)��3H2(g)2NH3(g)����H����92.4 kJ·mol��1���ڷ�Ӧ�����У���Ӧ���ʵı仯��ͼ��ʾ����������ʵı仯�ش��ȡ�Ĵ�ʩ��
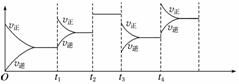
t1____________��t2______________��t3______________��
t4____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����������A��B��Ϊͬ���칹�壬����C��H��O����Ԫ�ء���ͬ״���£�A��B����������������ܶ���97��������C��Hԭ�Ӹ�����ͬ����C��Hԭ����֮����Oԭ������5����
��1��A�ķ���ʽ��________________����֪�����л�����������ת����ϵ
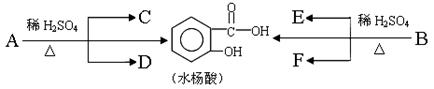 |
����C�ܷ���������Ӧ��F����������������C��C��D����Է���������ͬ�IJ�ͬ���͵��л��
��2��ˮ���������������ŵ�������____________����ȫȼ��ʱ��1 mol D��1 mol �����л����������ͬ����____________������ĸ���ţ���
a. C3H6O3 b. C3H8O c. C2H4 d. C2H6O2
��3��B�Ľṹ��ʽ��________________________��
ˮ������С�մ���Һ��Ӧ�Ļ�ѧ����ʽ��_________________________________�� һ�������£�C��F��Ӧ�ķ�Ӧ������____________��
��4��д��C������������Һ�ķ�Ӧ��ѧ����ʽ��________________________________����Ӧ������____________��
��5��ͬʱ��������Ҫ��Ļ�������____________�֡�
�� ��A��Ϊͬ���칹�� �� ����ˮ��
�� ������������ȡ�������ұ����ϵ�һ�ȴ���ֻ��1��
��6��1 mol������5���е�һ���л���X ��ˮ��Һ�У�����4 mol NaOH���ȷ�����Ӧ��д���˷�Ӧ�Ļ�ѧ����ʽ��____________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com