
(12��)ij��ѧ����С���о��Ҵ�������ʵ�鲢��֤��������˼ס�������װ��(ͼ�еļг�������δ��������������ʾ�ƾ�����Դ)��ÿ��װ���ֿɻ���Ϊ�١��ڡ��������֡�������ʢ�ŵ��Լ�Ϊ��a����ˮ�Ҵ�(�е㣺78��)��b��ͭ˿��c����ˮ����ͭ��d������������ͭ����Һ��


(1)�������������Ե��ŵ㣺
�ף�__________________________________________________________________��
�ң�___________________________________________________________________��
(2)�������������ŵ㣬���һ�ױȽϺ������Ƶ�ʵ��װ�ã��ɰ������������ҵ�˳���ʾΪ_________________________________________________________(����ע٣��Ң�)��
(3)��Ҫ��֤��ʵ���нϸߵ�Ч�ʣ����貹���������________������_______________��
(4)ʵ��������֤�Ҵ����������ʵ��������________________________________��
(5)װ���У�����ȥ�ڢٲ��֣������������䣬����ˮ����ͭ�����Ա仯������������(4)��ͬ���ƶ�ȼ�չ�����Ҫ��Ӧ�Ļ�ѧ����ʽ��__________________________________��
(1)�ף������÷�Һ©�����Կ���Һ������������Ʒ�Ӧ�Ľ��У���������ˮԡ���ȣ����γɽ�ƽ�ȵ��Ҵ�������ʹ��Ӧ����֣����е�б�������������������Ҵ����������á���2�֣�
�ң�������еĸ���ܿɷ�ֹ��Һ�е�ˮ����ˮ����ͭ��Ӧ������������������֤��2�֣�
(2)�Ң٣��עڣ��Ңۣ�2�֣�
(3)�¶ȼơ�����ˮԡ�¶���78����Ը���78�棬ʹ�Ҵ�����ƽ�����������ٻӷ�����߷�ӦЧ�ʡ���2�֣�
(4)c����ˮ����ͭ������d�����ɺ�ɫ������2�֣�
(5)C2H5OHCu��CH3CHO��H2����2�֣�
����������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||
| 0.36-(12.8-w)/32 |
| V��1��10 -3 |
| 0.36-(12.8-w)/32 |
| V��1��10 -3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012����У�Ϻ��У�����������ʦ������У�����������ѧ�Ծ����������� ���ͣ�ʵ����
�����12�֣�
ij��ѧ����С�����������ͼ��ʾ��װ�ý���ʵ�顣ͼ�м�ͷ��ʾ��������A��ʾһ�ִ�������������壬B����һ�����壬��Ӧ����һ��ʱ���װ�ü����к���ɫ�������ɡ�ʵ�������õ�ҩƷ�����ֻ�ܴ�����������ѡȡ��Na2CO3��NaHCO3��MnO2��Na2O2��NaCl����ˮCaCl2 ��NH4HCO3����ʯ�ҵȹ��������ˮ��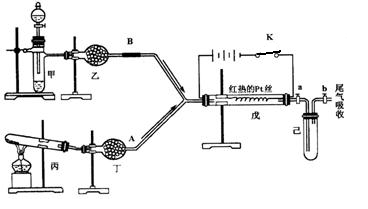
����ͼ��װ�úͷ�Ӧ������ش�
��1�����з�����Ӧ�Ļ�ѧ����ʽΪ ��
��2�����еĸ����Ӧѡ ________����ѡ��һ�ָ�������� ��
��3�����з�����Ӧ�Ļ�ѧ����ʽΪ ��
��4�����з�������Ҫ��Ӧ�Ļ�ѧ����ʽΪ____________________���˷�Ӧ��________�����ȡ����ȣ���Ӧ����֤������жϵ������� ��
��5�������г�������ɫ�����ֹͣ�������ȣ����ر�a��b���������������������ˮ�У������л���ֵ������ǣ�______________�������������ԭ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ������ʡ����һ�и߶���ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ�ʵ����
(12��)ij��ѧ����С���о��Ҵ�������ʵ�鲢��֤��������˼ס�������װ��(ͼ�еļг�������δ��������������ʾ�ƾ�����Դ)��ÿ��װ���ֿɻ���Ϊ�١��ڡ��������֡�������ʢ�ŵ��Լ�Ϊ��a����ˮ�Ҵ�(�е㣺78��)��b��ͭ˿��c����ˮ����ͭ��d������������ͭ����Һ��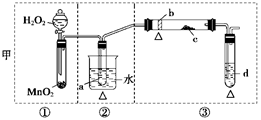

(1)�������������Ե��ŵ㣺
�ף�__________________________________________________________________��
�ң�___________________________________________________________________��
(2)�������������ŵ㣬���һ�ױȽϺ������Ƶ�ʵ��װ�ã��ɰ������������ҵ�˳���ʾΪ_________________________________________________________(����ע٣��Ң�)��
(3)��Ҫ��֤��ʵ���нϸߵ�Ч�ʣ����貹���������________������_______________��
(4)ʵ��������֤�Ҵ����������ʵ��������________________________________��
(5)װ���У�����ȥ�ڢٲ��֣������������䣬����ˮ����ͭ�����Ա仯������������(4)��ͬ���ƶ�ȼ�չ�����Ҫ��Ӧ�Ļ�ѧ����ʽ��__________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�갲��ʡ�����������¿���ѧ�Ծ��������棩 ���ͣ�ʵ����
(11��)ij��ѧ������ȤС��Ϊ̽��ͭ��Ũ����ķ�Ӧ������������ͼ��ʾװ�ý�����
��ʵ�飺

��1���ȹرջ���a����6.4 gͭƬ��12 mL 18 mol/L��Ũ�������Բ����ƿ�й�������Ӧ��ɣ�������ƿ�л���ͭƬʣ�࣮�ٴ���a���������е�������������Բ����ƿ�����ͭƬ��ȫ��ʧ��
��д��������������ƿ�ڷ�����Ӧ�Ļ�ѧ����ʽ��
����a֮ǰ ��
����a֮�� ��
��B�������ռ�ʵ���в����������װ�ã�������ƿ�ڵĵ���δ��ȫ����ֱ����ͼ�ϰѵ��ܲ���������
��2��ʵ���ϣ��ڴ���a֮ǰ��������ʣ�ࡣΪ�����ⶨ��������ʵ������ס�����ѧ��������������ƣ�
�ټ�ѧ����Ʒ����ǣ��Ȳⶨͭ��Ũ���ᷴӦ����SO2��������ͨ������ȷ����������ʵ��������ⶨSO2�ķ����ǽ�װ��A���������建��ͨ��װ��D���Ӷ����װ��A������������(������ɱ�״��)������Ϊ��ѧ����Ƶ�ʵ�鷽����Dװ�����Լ�Ϊ�� (�ѧʽ)��
����ѧ����Ƶķ����ǣ�����Ӧ�����Һ��ȴ��ȫ�����뵽�ձ���ϡ�ͣ�������ȷ��������100 mL��Һ����ȡ20 mL����ƿ�У��� ��ָʾ�����ñ�����������Һ���еζ�[��֪��Cu(OH)2��ʼ������pHԼΪ5]��ѡ���ָʾ��������Ϊ ���������������ʵ���������ȥa mol/L����������Һb mL����ԭ��������ʵ���Ϊ mol(�ú�a��b�ı���ʽ����ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ����У�Ϻ��У�����������ѧ�Ծ��������棩 ���ͣ�ʵ����
�����12�֣�
ij��ѧ����С�����������ͼ��ʾ��װ�ý���ʵ�顣ͼ�м�ͷ��ʾ��������A��ʾһ�ִ�������������壬B����һ�����壬��Ӧ����һ��ʱ���װ�ü����к���ɫ�������ɡ�ʵ�������õ�ҩƷ�����ֻ�ܴ�����������ѡȡ��Na2CO3��NaHCO3��MnO2��Na2O2��NaCl����ˮCaCl2 ��NH4HCO3����ʯ�ҵȹ��������ˮ��

����ͼ��װ�úͷ�Ӧ������ش�
��1�����з�����Ӧ�Ļ�ѧ����ʽΪ ��
��2�����еĸ����Ӧѡ ________����ѡ��һ�ָ�������� ��
��3�����з�����Ӧ�Ļ�ѧ����ʽΪ ��
��4�����з�������Ҫ��Ӧ�Ļ�ѧ����ʽΪ____________________���˷�Ӧ��________�����ȡ����ȣ���Ӧ����֤������жϵ������� ��
��5�������г�������ɫ�����ֹͣ�������ȣ����ر�a��b���������������������ˮ�У������л���ֵ������ǣ�______________�������������ԭ�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com