
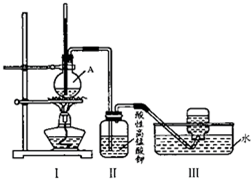
 ijѧϰС����ʵ���������Ҵ���Ũ�����ϼ�������ϩ���������й���ϩ���ʵ�̽����װ����ͼ��
ijѧϰС����ʵ���������Ҵ���Ũ�����ϼ�������ϩ���������й���ϩ���ʵ�̽����װ����ͼ��| Ũ���� |
| 170�� |
| Ũ���� |
| 170�� |


 �������ϵ�д�
�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ӱ�ʡ�������и߶���ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ�ʵ����
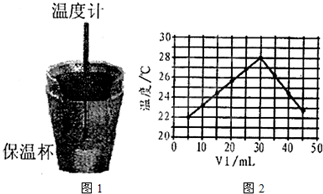
(10��)ij��ѧѧϰС����ʵ������������ͼװ�òⶨ�кͷ�Ӧ�е���ЧӦ��ʵ��ʱ�� ��Һ��
��Һ�� δ֪Ũ�ȵ�NaOH��Һ��Ͼ��Ⱥ�������¼��Һ�¶ȣ�����ʵ����������ͼ��ʾ(ʵ����ʼ�ձ���
δ֪Ũ�ȵ�NaOH��Һ��Ͼ��Ⱥ�������¼��Һ�¶ȣ�����ʵ����������ͼ��ʾ(ʵ����ʼ�ձ��� )��
)��
�ݴ���ش��������⣺ (1)��ʵ��װ���Ͽ�����ͼ����ȱ�ٵ�һ�ֲ�����Ʒ��________________________��
(1)��ʵ��װ���Ͽ�����ͼ����ȱ�ٵ�һ�ֲ�����Ʒ��________________________��
(2)���±���������_______________________________________________________��
(3)ijͬѧ�����������ݣ���������¹۵㣬������ȷ����______________________��
A������ʵ��ʱ�����¶�Ϊ22��
B����ʵ�������ѧ�ܿ���ת��Ϊ����
C����ʵ�������ˮ���ɵķ�Ӧ���Ƿ��ȷ�Ӧ
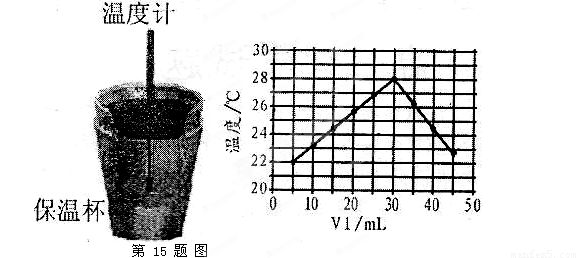
(4)����ͼ���������ݣ��ɲ��NaOH��Һ��Ũ��ԼΪ______________
(5)����ʹ�ñ��±��⣬Ϊ�˱�֤ʵ��ɹ�����Ҫע����Щ����(˵��1�㼴
��) ____________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ӱ�ʡ�߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
(10��)ij��ѧѧϰС����ʵ������������ͼװ�òⶨ�кͷ�Ӧ�е���ЧӦ��ʵ��ʱ��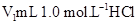 ��Һ��
��Һ��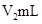 δ֪Ũ�ȵ�NaOH��Һ��Ͼ��Ⱥ�������¼��Һ�¶ȣ�����ʵ����������ͼ��ʾ(ʵ����ʼ�ձ���
δ֪Ũ�ȵ�NaOH��Һ��Ͼ��Ⱥ�������¼��Һ�¶ȣ�����ʵ����������ͼ��ʾ(ʵ����ʼ�ձ���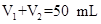 )��
)��
�ݴ���ش��������⣺
 (1)��ʵ��װ���Ͽ�����ͼ����ȱ�ٵ�һ�ֲ�����Ʒ��________________________��
(1)��ʵ��װ���Ͽ�����ͼ����ȱ�ٵ�һ�ֲ�����Ʒ��________________________��
(2)���±���������_______________________________________________________��
(3)ijͬѧ�����������ݣ���������¹۵㣬������ȷ����______________________��
A������ʵ��ʱ�����¶�Ϊ22��
B����ʵ�������ѧ�ܿ���ת��Ϊ����
C����ʵ�������ˮ���ɵķ�Ӧ���Ƿ��ȷ�Ӧ
(4)����ͼ���������ݣ��ɲ��NaOH��Һ��Ũ��ԼΪ______________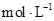
(5)����ʹ�ñ��±��⣬Ϊ�˱�֤ʵ��ɹ�����Ҫע����Щ����(˵��1�㼴
��) ____________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(12��)ij��ѧѧϰС����ʵ������������ͼװ�òⶨ�кͷ�Ӧ�е���ЧӦ��ʵ��ʱ��![]() ��Һ��
��Һ��![]() δ֪Ũ�ȵ�NaOH��Һ��Ͼ��Ⱥ�������¼��Һ�¶ȣ�����ʵ����������ͼ��ʾ(ʵ����ʼ�ձ���
δ֪Ũ�ȵ�NaOH��Һ��Ͼ��Ⱥ�������¼��Һ�¶ȣ�����ʵ����������ͼ��ʾ(ʵ����ʼ�ձ���![]() )��
)��
�ݴ���ش��������⣺
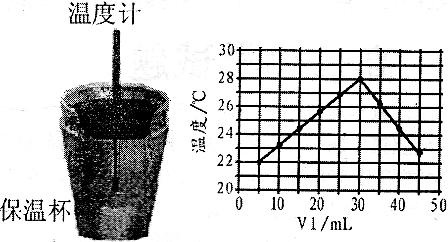
�� 15 �� ͼ
(1)��ʵ��װ���Ͽ�����ͼ����ȱ�ٵ�һ�ֲ�����Ʒ��________________________��
![]() (2)���±���������_______________________________________________________��
(2)���±���������_______________________________________________________��
(3)ijͬѧ�����������ݣ���������¹۵㣬������ȷ����______________________��
A������ʵ��ʱ�����¶�Ϊ22��
B����ʵ�������ѧ�ܿ���ת��Ϊ����
C����ʵ�������ˮ���ɵķ�Ӧ���Ƿ��ȷ�Ӧ
(4)����ͼ���������ݣ��ɲ��NaOH��Һ��Ũ��ԼΪ______________![]()
(5)����ʹ�ñ��±��⣬Ϊ�˱�֤ʵ��ɹ�����Ҫע����Щ����(˵��2�㼴
��) ____________________________��____________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com