



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��SO2��SiO2��CO��P2O5������������ |
| B��ϡ���������ᡢ�Ȼ�����Һ��Ϊ���� |
| C���������������֡�ˮ��������ˮ��Ϊ����� |
| D���ռ�����ᡢ���Ȼ�̼��ʯī��Ϊ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�����ʯ�л������뱥��ʳ��ˮ������ȡC2H2 |
| B����NH4HCO3������Һ�����������м������ɣ�����ȡNH4HCO3���� |
| C����FeCl3������Һ�������������ˮ���ȣ�����ȡFe��OH��3���� |
| D����CuCl2��Һ�����������м������ɣ�����ȡ��ˮCuCl2���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A | B | C | D |
 |
 |
 |
 |
| ��֤��ѧ��ת��Ϊ���� | ��֤�¶ȶ�ƽ���ƶ���Ӱ�� | ��֤�������ⸯʴ | ��֤�ǽ���Cl��C��Si |
| A��A | B��B | C��C | D��D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
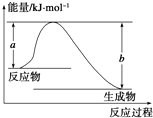 �Ͽ�1mol AB��g�������еĻ�ѧ��ʹ��ֱ�������̬Aԭ�Ӻ���̬Bԭ�������յ�������ΪA-B���ļ��ܣ��±��г���һЩ��ѧ���ļ���E��
�Ͽ�1mol AB��g�������еĻ�ѧ��ʹ��ֱ�������̬Aԭ�Ӻ���̬Bԭ�������յ�������ΪA-B���ļ��ܣ��±��г���һЩ��ѧ���ļ���E��| ��ѧ�� | H-H | Cl-Cl | O�TO | C-Cl | C-H | O-H | H-Cl |
| E/kJ?mol-1 | 436 | 247 | x | 330 | 413 | 463 | 431 |
| 1 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��1���ö��Ե缫���CuSO4��Һ����ͼ��װ�ã����ù����е��ص缫��ӦʽΪ��������
��1���ö��Ե缫���CuSO4��Һ����ͼ��װ�ã����ù����е��ص缫��ӦʽΪ���������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ѡ�� | ʵ�� | ���ͻ���� |
| A | �ýྻ��Ptպȡij��Һ������ɫ��Ӧ������ʻ�ɫ | ����Һ��һ������Na+����k+ |
| B | �ýྻ�IJ����������Na2O2����֬��������֬��ȼ�� | CO2��H2O��Na2O2��Ӧ�Ƿ��ȷ�Ӧ |
| C | ����ˮ�е���ֲ���ͣ����Ͳ�����ɫ | �岻������֬ |
| D | �������ữ��H2O2����Fe��NO3��2��Һ����Һ���ɫ | H2O2�������Ա�Fe3+ǿ |
| A��A | B��B | C��C | D��D |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com