
A.¹¤ŅµÉś²śÓ¦ŌŚ½ĻµĶµÄĪĀ¶ČĻĀ½ųŠŠ
B.·“Ó¦ÖŠĪüŹÕČČĮæĪŖ2.55A kJ
C.Ę½ŗāŹ±»ģŗĻĘųĢåÖŠĢ¼”¢ĒāŌŖĖŲµÄÖŹĮæ±Č1£ŗ6
D.Ę½ŗāŹ±ČŻĘ÷ÄŚµÄŃ¹ĒæĪŖæŖŹ¼Ź±µÄ0.55±¶

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 Ņ»¶ØĢõ¼žĻĀ£¬ŌŚĢå»żĪŖ3LµÄĆܱÕČŻĘ÷ÖŠ£¬Ņ»Ńõ»ÆĢ¼ÓėĒāĘų·“Ӧɜ³É¼×“¼£Ø“߻ƼĮĪŖCu2O/ZnO£©£ŗCO£Øg£©+2H2£Øg£©?CH3OH£Øg£©
Ņ»¶ØĢõ¼žĻĀ£¬ŌŚĢå»żĪŖ3LµÄĆܱÕČŻĘ÷ÖŠ£¬Ņ»Ńõ»ÆĢ¼ÓėĒāĘų·“Ӧɜ³É¼×“¼£Ø“߻ƼĮĪŖCu2O/ZnO£©£ŗCO£Øg£©+2H2£Øg£©?CH3OH£Øg£©| c(CH3OH) |
| c(CO)?C2(H2) |
| c(CH3OH) |
| c(CO)?C2(H2) |
| 2nB |
| 3tB |
| 2nB |
| 3tB |
| 1 |
| 2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
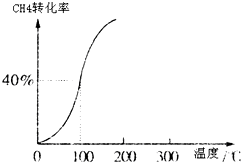 ¢ń”¢ŗĻ³É°±¶ŌÅ©ŅµÉś²ś¼°¹ś·Ą½ØÉč¾ł¾ßÓŠÖŲŅŖŅāŅ壮
¢ń”¢ŗĻ³É°±¶ŌÅ©ŅµÉś²ś¼°¹ś·Ą½ØÉč¾ł¾ßÓŠÖŲŅŖŅāŅ壮| 1 | 2 |
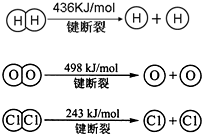
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
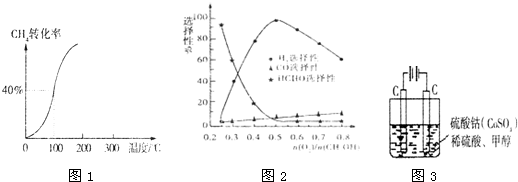
| 1 |
| 2 |
| c(H2) |
| c(CH3OH) |
| “߻ƼĮ |
| ¼ÓČČ |
| “߻ƼĮ |
| ¼ÓČČ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
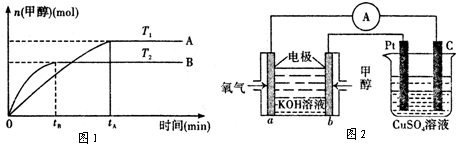
| “߻ƼĮ | ¼ÓČČ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com