
����ͼ��ʾװ�ý���ʵ�飬��Һ��A��μ��뵽����B�У��ش��������⣺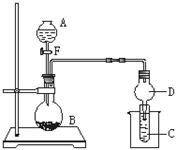
��ͼ��Dװ����ʵ���е������� ��
���� AΪ30%H2O2��Һ��BΪMnO2 ��Cʢ�������ᣨH2S�� ������Һ������E��C�г���dz��ɫ���ǵ�����д��C�з�����Ӧ�Ļ�ѧ����Ϊ ��
����AΪŨ���ᣬBΪKMnO4��C��ʢ��KI������Һ������E��C�е����� ������ͨ������C�У��㹻����ʱ�����C����Һ����ɫ��ʧ��������Ϊ����Һ��I2�ܱ�Cl2����ΪHIO3��д���÷�Ӧ�����ӷ�Ӧ����ʽ ��
����AΪŨ��ˮ��BΪ��ʯ�ң�C��ʢ��AlCl3��Һ������E���㹻����ʱ���C�е������� ��C�з�����Ӧ�����ӷ���ʽΪ ��
(5)��BΪ��״����ʯ��CΪ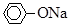 ��Һ��ʵ���й۲쵽��Һ����ǣ�����A���������е�________��
��Һ��ʵ���й۲쵽��Һ����ǣ�����A���������е�________��
A.HCl B.HNO3 C.H2SO4 D.CH3COOH

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
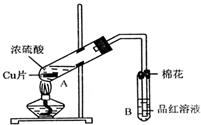
 ij�о�С������ͼ��ʾװ�ý���ͭ��Ũ���ᷴӦ��ʵ���о���
ij�о�С������ͼ��ʾװ�ý���ͭ��Ũ���ᷴӦ��ʵ���о���
| ||
| ||
| �������� | �����뾧��������� | ���Ⱥ���������������� |
| 11.7g | 22.7g | 18.9g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���ӱ�ʡ������ѧ2010�������ѧ�ڵ�һ���¿���ѧ ���ͣ�058
ij�о�С������ͼ��ʾװ�ý���ͭ��Ũ���ᷴӦ��ʵ���о���
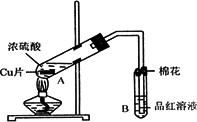
(1)д���Թ�B�е�ʵ������________��
(2)д��A�з�Ӧ�Ļ�ѧ����ʽ________
(3)������A�Թ��м���H2O2������ͭƬ�ܽ⣬��Ӧ�����ӷ���ʽΪ��________�����Բ�����Ũ���ᣬֻҪ��ʹͭƬ�ܽ⣬Ҳ���Լ���(��д�������ڲ�ͬ������ʵĻ�ѧʽ)________��________��
(4)B�Թܿڵ���Ӧմ�е��Լ���________��
(5)С���Ա��Ӧ�����Һ�м�������������ͭ��ʹʣ�������ȫ��ת��Ϊ����ͭ�����˺���Һ����Ũ������ȴ�ᾧ�Ƶ�����ͭ����(CuSO4��xH2O)��С���Ա���ü��ȷ��ⶨ�þ�����ᾧˮx��ֵ��
�������ǵ�ʵ������У����ٳ���________�Σ�
������������һ��ʵ������ݣ�
�����ϱ����ݼ����ж�x��ʵ��ֵ������ֵ(x��5)________(�ƫ����ƫС��)�����ʵ���в�������ԭ�������________(�����)
A������ͭ�����к��в��ӷ�������
B��ʵ��ǰ���������ʪ��ˮ
C������ʱ�о���ɽ���ȥ
D������ʧˮ��¶���ڿ�������ȴ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
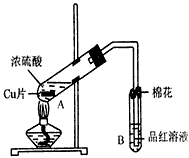
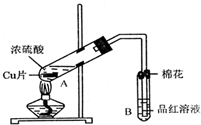 ij�о�С������ͼ��ʾװ�ý���ͭ��Ũ���ᷴӦ��ʵ���о���
ij�о�С������ͼ��ʾװ�ý���ͭ��Ũ���ᷴӦ��ʵ���о���| �������� | �����뾧��������� | ���Ⱥ���������������� |
| 11.7g | 22.7g | 18.9g |
�鿴�𰸺ͽ���>>
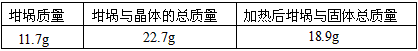
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
| �������� | �����뾧��������� | ���Ⱥ���������������� |
| 11.7g | 22.7g | 18.9g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��0103 �¿��� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com