
| 1 | 2 |
| 1 |
| 2 |
| 1 |
| 2 |


 �¿α�����Ķ�ѵ��ϵ�д�
�¿α�����Ķ�ѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Ԫ�� | �����Ϣ |
| X | X�Ļ�̬ԭ�Ӻ���3���ܼ����е��ӣ���ÿ���ܼ��ϵĵ�������� |
| Y | ԭ�������������Ǵ��������� |
| Z | ���ʼ��仯�������ɫ��ӦΪ��ɫ |
| W | WԪ�ػ�̬ԭ�ӵ�M��ȫ������N��ֻ��һ������ |
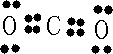
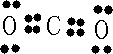
| 1 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �¶ȣ��棩 | 20 | 30 | 40 | 50 | 60 | 70 | 80 |
| ͭƽ���ܽ����� ����10-3 mol?L-1?min-1�� | 7.34 | 8.01 | 9.25 | 7.98 | 7.24 | 6.73 | 5.76 |
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| 17Cv |
| 200�� |
| 17Cv |
| 200�� |
| ʵ������ | ʵ�鷽�� | ʵ������ |
| ��֤������ | ȡ�����⻯�ص�����Һ��ϡ�������Թ��У��������������Һ������ȡ��������������Һ���Թ��У��������������Һ���� | ��Һ����ɫ ��Һ����ɫ ������ ��������ɫ��������Һ����� ��������ɫ��������Һ����� �� |
| ��֤�Ȳ��ȶ��� | ȡ��������������Һ���Թ��У� ������ ������ �ô����ǵ�ľ������ �ô����ǵ�ľ������ �� |
�������ݣ������ǵ�ľ����ȼ�� |
| �¶ȣ��棩 | 20 | 30 | 40 | 50 | 60 | 70 | 80 |
| ͭƽ���ܽ����� ����10-3mol?L-1?min-1�� |
7.34 | 8.01 | 9.25 | 7.98 | 7.24 | 6.73 | 5.76 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 1 | 2 |
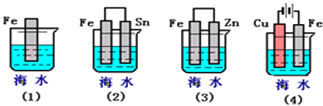
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com