
| 1 |
| 3 |
| 1 |
| 3 |
| n |
| v |


 轻松课堂单元期中期末专题冲刺100分系列答案
轻松课堂单元期中期末专题冲刺100分系列答案科目:高中化学 来源:不详 题型:单选题
| A.通入酸性KMnO4溶液中 | B.通入足量的溴水中 |
| C.点燃 | D.能入H2后加热 |
查看答案和解析>>
科目:高中化学 来源:不详 题型:问答题
| 实验编号 | 邻苯二甲酸氢钾的质量(g) | 待测NaOH溶液的体积(mL) |
| 1 | 0.4162 | 18.25 |
| 2 | 17.04 | |
| 3 | 16.96 |

查看答案和解析>>
科目:高中化学 来源:不详 题型:问答题
| V(HCl)/mL | 0.00 | 12.00 | 18.00 | 22.00 | 23.00 | 23.96 | 24.00 | 24.04 | 25.00 | 26.00 | 30.00 |
| pH | 13.1 | 12.6 | 12.2 | 11.7 | 11.4 | 9.9 | 7.0 | 4.0 | 2.7 | 2.4 | 1.9 |
| 指示剂 | 变色范围 (pH) | 各范围内颜色 | ||
| 前 | 中间 | 后 | ||
| 甲基橙 | 3.1~4.4 | 红 | 橙色 | 黄 |
| 石蕊 | 5.0~8.0 | 红 | 紫色 | 蓝 |
| 酚酞 | 8.2~10.0 | 无 | 粉红 | 红 |
查看答案和解析>>
科目:高中化学 来源:不详 题型:问答题
查看答案和解析>>
科目:高中化学 来源:不详 题型:填空题
| 滴定次数 | NaOH溶液体积(mL) | |
| V1 | V2 | |
| 1 | 3.05 | 44 |
| 2 | 1.45 | 41.5 |
| 3 | 7.65 | 47.6 |
查看答案和解析>>
科目:高中化学 来源:不详 题型:问答题

查看答案和解析>>
科目:高中化学 来源:不详 题型:问答题
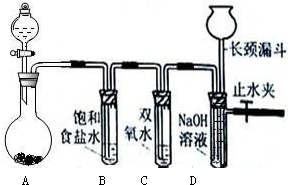
查看答案和解析>>
科目:高中化学 来源:不详 题型:单选题
| A.实验室制备NH3可用NaOH固体和浓氨水为原料方便制得 |
| B.二氧化硅是生产光纤制品的基本原料 |
| C.工业制水泥、玻璃、陶瓷都要用到石灰石为原料 |
| D.工业上可利用铝热反应原理制备高熔点金属 |
查看答案和解析>>
湖北省互联网违法和不良信息举报平台 | 网上有害信息举报专区 | 电信诈骗举报专区 | 涉历史虚无主义有害信息举报专区 | 涉企侵权举报专区
违法和不良信息举报电话:027-86699610 举报邮箱:58377363@163.com