
| A��һ��û��N2��CO��HCl��������һ�� |
| B��һ��û��N2��CO��HCl���� |
| C��һ����N2��CO��HCl��������һ�� |
| D��һ����N2��HCl���϶�û��CO |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
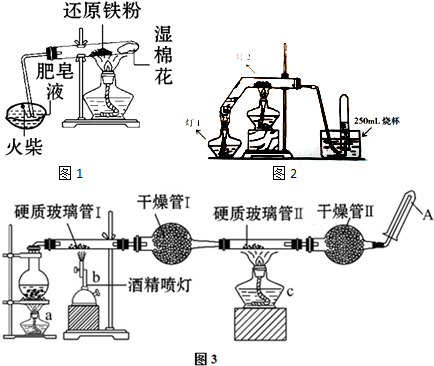
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
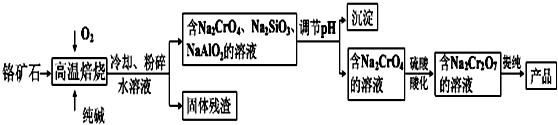
| 3 | 0.4 |
| 3 | 4 |
| 3 | 6 |
| 3 | 60 |
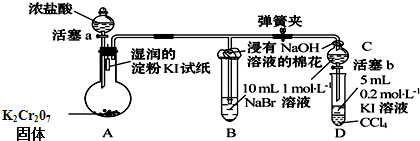
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ʵ��������480mL 0.05mol/L��FeSO4��Һ��ʵ����������У�
ʵ��������480mL 0.05mol/L��FeSO4��Һ��ʵ����������У��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��NaHSˮ�ⷴӦ��HS-+H2O�TH3O++S2- | ||||
B�����������ȵ�Ũ���ᷴӦ��Fe+6HNO3��Ũ��
| ||||
C�� ��ͼװ����ʯī�缫�ϵĵ缫��ӦΪ��O2+4e-+2H2O=4OH- | ||||
D��ʪ����ͭ��ԭ����Cu2S+O2
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����ˮ���ܽ��ԣ�Na2CO3��NaHCO3 |
| B�����ȶ��ԣ�Na2CO3��NaHCO3 |
| C��ͬŨ����Һ�������ᷴӦ���ٶȣ�Na2CO3��NaHCO3 |
| D����������������ˮ�ų���������Na2CO3��NaHCO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com