

���� ��1����һ��ˮ�����ɵ�H3O+�Եڶ���ˮ�����������ã���һ��ˮ��Ϊ����
��2���������ƵĻ�ԭ����Ϊ�Ȼ��ƣ���������������ˮ���ɣ��Ȼ��ơ�ˮ�Ի������Ѻã����ԭ���غ���ƽ��д�õ���ѧ����ʽ��
��3����ͼ���������¶ȣ�ƽ����ϵ�������������������ͣ���ƽ�������ƶ���˵�������������ȷ�Ӧ�����ڴ��������·ֽ�ֻ�����������壬����һ��������ʹ��ɫʯ����ֽ����ɫ����ʽΪ��3N2H4$\stackrel{����}{?}$4NH3+N2������ѹǿ��ƽ�������ƶ���N2H4�����������P1����P2��
��4���ٷ�ӦI�����ȷ�Ӧ����ӦII�Ƿ��ȷ�Ӧ��
�ڸ��ݸ�˹����֪����ӦI��7+��ӦII��4=��ӦIII��
����ƽ��ʱn��N2��=a��n��H2��=2a��
n��N2H4��=0.1 mol-a����3a=3.0����0.1 mol-a����a=0.05 mol��
�ԣ�H2��=$\frac{��c}{��t}$��K=$\frac{c��{N}_{2}����{c}^{2}��{H}_{2}��}{c��{N}_{2}{H}_{4}��}$���㣻
��5��N2H4/�����ڼ���Һ�й��ɵ�أ�N2H4�ڸ����Ϸ���������Ӧ��O2�������Ϸ�����ԭ��Ӧ�������ĵ缫��ӦʽΪN2H4-4e-+4OH-=N2��+4H2O������ʱ�����ķ�ӦʽΪNi2++2e-=Ni���������ԭ������Ϊ59������Ϊ�����������ĵ缫��ӦʽΪNi-2e-=Ni2+�����ݵ�ʧ�����غ㽨����ϵ���㣮
��� �⣺��1����ͬ�¶��£�����ƽ�ⳣ��K2��K1������Ҫԭ���ǵ�һ��ˮ�����ɵ�H3O+�Եڶ���ˮ�����������ã���һ��ˮ��Ϊ����
�ʴ�Ϊ����һ��ˮ�����ɵ�H3O+�Եڶ���ˮ�����������ã�
��2���������ƵĻ�ԭ����Ϊ�Ȼ��ƣ���������������ˮ���ɣ��Ȼ��ơ�ˮ�Ի������Ѻã���ѧ����ʽΪ��NaClO+2NH3=NaCl+N2H4+H2O��
�ʴ�Ϊ��NaClO+2NH3=NaCl+N2H4+H2O��
��3����ͼ���������¶ȣ�ƽ����ϵ�������������������ͣ���ƽ�������ƶ���˵�������������ȷ�Ӧ�����ڴ��������·ֽ�ֻ�����������壬����һ��������ʹ��ɫʯ����ֽ����ɫ����ʽΪ��3N2H4$\stackrel{����}{?}$4NH3+N2������ѹǿ��ƽ�������ƶ���N2H4�����������P1����P2��
�ʴ�Ϊ����������
��4���ٷ�ӦI�����ȷ�Ӧ����H1��0����ӦII�Ƿ��ȷ�Ӧ����H2��0�����H1����H2��
�ʴ�Ϊ������
�ڷ�ӦI��N2H4��g��?N2��g��+2H2��g����H1
��ӦII��N2��g��+3H2��g��?2NH3��g����H2
��ӦIII��7N2H4��g��?8NH3��g��+3N2��g��+2H2��g����H
���ݸ�˹����֪����ӦI��7+��ӦII��4=��ӦIII����H=7��H1+4��H2��
�ʴ�Ϊ��7��H1+4��H2��
����ƽ��ʱn��N2��=a��n��H2��=2a��
n��N2H4��=0.1 mol-a����3a=3.0����0.1 mol-a����a=0.05 mol��
�ԣ�H2��=$\frac{0.05mol��2}{1L��4min}$=0.025mol/��L•min����K=$\frac{c��{N}_{2}����{c}^{2}��{H}_{2}��}{c��{N}_{2}{H}_{4}��}$=$\frac{\frac{0.05}{1}����\frac{0.05��2}{1}��^{2}}{0.1-0.05}$=0.01��
�ʴ�Ϊ��0.025��0.01��
��5��N2H4/�����ڼ���Һ�й��ɵ�أ�N2H4�ڸ����Ϸ���������Ӧ��O2�������Ϸ�����ԭ��Ӧ�������ĵ缫��ӦʽΪN2H4-4e-+4OH-=N2��+4H2O��
����ʱ�����ķ�ӦʽΪNi2++2e-=Ni���������ԭ������Ϊ59������Ϊ�����������ĵ缫��ӦʽΪNi-2e-=Ni2+������������N2H4�����ʵ���Ϊn���ɵ����غ�֪��������������������֮��Ϊ[2n-��-2n��]��59g/m/ol=1.18g��n=0.005mol��m��N2H4��=0.005mol��32g/mol=0.16g��
�ʴ�Ϊ��N2H4-4e-+4OH-=N2��+4H2O��0.16��
���� ���⿼���Ϊ�ۺϣ��漰��Ӧ���ʡ�ƽ�ⳣ����ƽ���ƶ�����˹�����Լ��绯ѧ��֪ʶ����Ŀ�Ѷ��еȣ�ע�����Ӱ��ƽ���ƶ��������Լ�ƽ���ƶ�������жϣ�


 Сѧ��10���ӿ������100��ϵ�д�
Сѧ��10���ӿ������100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | �Ҵ���Ũ���ᣬ���ȵ�170��ʱ�ϼ��ڢ� | |
| B�� | �Ҵ���Ũ���ᣬ���ȵ�140��ʱ�ϼ��٢� | |
| C�� | �Ҵ��ͽ����Ƶķ�Ӧ�ϼ��� | |
| D�� | �Ҵ���Cu������O2��Ӧʱ�ϼ��٢� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
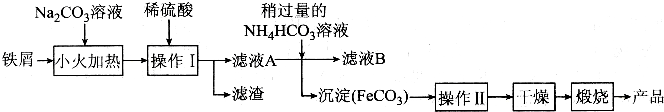
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢ڢۢ� | B�� | �٢ۢݢޢ� | C�� | �ڢۢݢ� | D�� | �ڢۢܢݢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 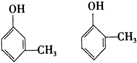 | B�� | 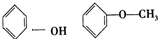 | ||
| C�� | 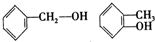 | D�� | 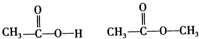 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
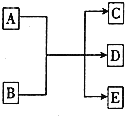 ��һ�������£�����A��E��ת����ϵ��ͼ��ʾ������AΪ���ʣ�������EΪ��ɫҺ�壮
��һ�������£�����A��E��ת����ϵ��ͼ��ʾ������AΪ���ʣ�������EΪ��ɫҺ�壮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢ڢܢ� | B�� | �ڢۢݢ� | C�� | �٢ܢݢ� | D�� | �٢ۢܢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
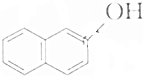 �����л������У�����2-�ηӵ�ͬ���칹����ǣ�������
�����л������У�����2-�ηӵ�ͬ���칹����ǣ�������| A�� | 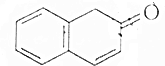 | B�� | 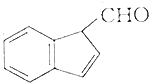 | ||
| C�� | 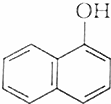 | D�� | 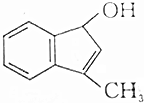 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ���������ӵİ뾶��������ԭ�Ӱ뾶 | |
| B�� | ���������ӵİ뾶С������ԭ�Ӱ뾶 | |
| C�� | �ǽ��������ӵİ뾶С����ԭ�Ӱ뾶 | |
| D�� | ��������Ų���ͬ�IJ�ͬ�����˵����Խ��뾶ԽС |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com