
������A��B����ѧ���������ʣ�������������ֻ�ܴ��±���ѡ��
| ������ | K����Na����Fe2����Ba2����NH |
| ������ | OH����NO |
(1)��A��B��ˮ�� Һ��Ϊ��ɫ����A��ˮ��Һ��ǿ���ԣ�B��ˮ��Һ��ǿ���ԡ���Ϻ����������ϡ����İ�ɫ��������ʹʪ��ĺ�ɫʯ����ֽ����ɫ�����塣
Һ��Ϊ��ɫ����A��ˮ��Һ��ǿ���ԣ�B��ˮ��Һ��ǿ���ԡ���Ϻ����������ϡ����İ�ɫ��������ʹʪ��ĺ�ɫʯ����ֽ����ɫ�����塣
��B�Ļ�ѧʽΪ__________________________________________________________��
��A��B��Һ��ϼ��ȷ�Ӧ�����ӷ���ʽ_____________________________________��
(2)��A��ˮ��Һ��dz��ɫ��B��ˮ��Һ��ɫ������ɫ��ӦΪ��ɫ����A��ˮ��Һ�м���ϡ���������������ټ���B����Һ��ƣ���A��B��ˮ��Һ����������Ա仯����
��A�Ļ�ѧʽΪ__________________________________________________________��
�ھ��� ��������������Һ��Ƶ�ԭ�����������(�����ӷ���ʽ��ʾ)
��������������Һ��Ƶ�ԭ�����������(�����ӷ���ʽ��ʾ)
��________________________________________________________________________��
��________________________________________________________________________��
������һ������֤��������Һ��Ƶ�ԭ��___________________________________��
 ������ϵ�д�
������ϵ�д� �±�Сѧ��Ԫ�Բ���ϵ�д�
�±�Сѧ��Ԫ�Բ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵����ȷ���ǣ� ��
A������λ�ڵ������ڵڢ�B��Ԫ��, ��һ����Ҫ�Ĺ���Ԫ��
B��������������������������������ɵĻ����
C��14�����ۺ�7����ۻ�Ϻ�����³�ַ�Ӧ������21��������
D�������������е�ȼ������FeBr3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪��A��B��C��D��E��F�ڳ����¾�Ϊ���壬����������ת����ϵ�����ַ�Ӧ��������������ȥ����
 |
��ش��������⣺
��1����A��ȼ�����뵽װ��E�ļ���ƿ�У��ɹ۲쵽��������_________________.
��2��C��Ũ��Һ���ɫ��ĩ��Ӧ����E�Ļ�ѧ����ʽΪ________________________��
��3��������Һ����뵽�⻯����Һ�У�������Ӧ�����ӷ���ʽΪ__________________����Ӧ�����Һ�м�������CCl4��������ã����Թ۲쵽��������_______________��
��4������Һ��Ƚϣ���Һ���ж���������� ���ѧʽ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����һƿ��ǩ��ע��Ϊ����������(�ơ�þ���ơ���)�ĸ����Ƽ���ijͬѧΪ��ȷ����ɷ֣�ȡ�����Ƽ���Ϊ��Һ����Ʋ����������ʵ�飺
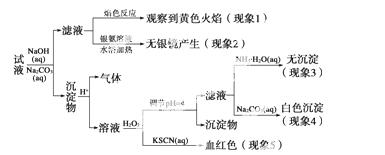
��֪��������ҺpH��4ʱ��Fe(OH)3������ȫ��Ca2����Mg2����������
��ͬѧ�ó��Ľ�����ȷ����  (����)
(����)
A����������1���Ƴ�����Һ�к���Na��
B����������2���Ƴ�����Һ�в����������������
C����������3��4���Ƴ�����Һ�к���Ca2������û��Mg2��
D����������5���Ƴ�����Һ��һ������Fe2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪����ǿ�������Һ���ֱ������������������еĸ�һ�֣����һ����ظ���NH4����Ba2����Na����H����SO42����NO3����OH����CO32��������������Һ�ֱ���ΪA��B��C��D����������ʵ�飺
����A��D�е���C�����г������ɣ���D��B��Ӧ���ɵ������ܱ�A���գ���A��D��Ӧ���ɵ������ܱ�B���ա�
�Իش��������⣺
(1)D�Ļ�ѧʽ��________���ж�������__________________________��
(2)д�����༸�����ʵĻ�ѧʽ��A________��B________��C________��
(3)д��ʵ������йط�Ӧ�����ӷ���ʽ____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪ij��Һ�д��ڽ϶��H����SO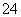 ��NO
��NO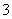 �������Һ�л����ܴ������ڵ���������(����)
�������Һ�л����ܴ������ڵ���������(����)
A��Al3����CH3COO����Cl���������� B��Na����NH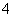 ��Cl��
��Cl��
C��Mg2����Cl����Ag�� D��Mg2����Ba2����Cl��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ӷ���ʽ�������
A����Ba(OH)2��Һ�еμ�ϡ���Ba2����2OH����2H���� SO42 �� =BaS04����2H2O
B�����Խ�����KMnO4���� H2O2��2MnO4�� ��5H2O2��6H�� = 2Mn2����5O2���� 8H2O
C�������ʵ� ����MgCl2��Ba(OH)2 �� HC1 ��Һ��ϣ�Mg2����2OH��= Mg(OH)2��
����MgCl2��Ba(OH)2 �� HC1 ��Һ��ϣ�Mg2����2OH��= Mg(OH)2��
D��Ǧ�����س��ʱ��������Ӧ��PbSO4��2H2O��2e- = PbO2��4H����SO42
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ط�Ӧ�����ӷ���ʽ��д��ȷ���� (����)
A������������������Fe(OH)3��3H��=Fe3����3H2O
B������ͭ��Һ�����ԣ�Cu2����2H2O=Cu(OH)2����2H��
C����̼�������Һ�мӹ���ʯ��ˮ�����ȣ�NH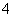 ��OH��=NH3����H2O
��OH��=NH3����H2O
D�����ữ�ĸ��������Һ����˫��ˮ��2MnO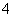 ��6H����5H2O2=2Mn2����5O2����8H2O
��6H����5H2O2=2Mn2����5O2����8H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
NAΪ�����ӵ���������ֵ������˵����ȷ����(����)
A��һ�������£�1.5 mol H2��0.5 mol N2��ַ�Ӧ��ɵõ�NH3�ķ�����ΪNA
B��������Fe��Cl2��Ӧ����0.1 mol����ʱʧȥ�ĵ�����Ϊ0.3NA
C�����³�ѹ�£�18 g H2O���еĵ�������Ϊ8NA
D����״���£�22.4 L�ļ�ϩ�к��еķ�����ΪNA
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com