
(12��)��ͼ��ʾ����ͼΪA�� E���ֺ�ͬ��Ԫ�ص������ת���Ĺ�ϵͼ������A��B��C��D�����¶������壬D�ʺ���ɫ����Ҫ��ش��������⣺
(1) A���ʵĵ���ʽ ��B�����Ǻ� ��
�� ���ӣ�����ԡ����ߡ��Ǽ��ԡ���
(2)���з�Ӧ�Ļ�ѧ����ʽΪ��
��B��C��
��D��E��
(3)ʵ������ȡB�Ļ�ѧ����ʽΪ�� ��ͨ���� ������B���壻
��֪B���������ȵ�����ͭ��Ӧ�õ�A����ͽ���ͭ����÷�Ӧ�Ļ�ѧ����ʽΪ�� ��
 ������ʱͬ����ϰ��ϵ�д�
������ʱͬ����ϰ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��1��������һ�ָ�Ч��������Դ��0.25mol������ȫȼ������Һ̬ˮʱ���ų�222.5kJ�����������ȼ���ȵ��Ȼ�ѧ��Ϊ
��1��������һ�ָ�Ч��������Դ��0.25mol������ȫȼ������Һ̬ˮʱ���ų�222.5kJ�����������ȼ���ȵ��Ȼ�ѧ��Ϊ| 1 |
| 2 |
| 1 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
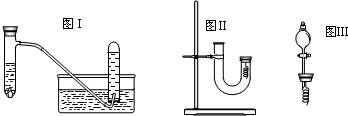
| ʵ�鲽�� | ���� |
| 1��U����˼���ϡ����ֱ������U���Ҷ� | ��/ |
| 2�ø���ͭ˿�Ľ�����סU���Ҷˣ��۲����� | ������ ����ɫ����������ұ���Һ�����ɫ ����ɫ����������ұ���Һ�����ɫ |
| 3����Ӧֹͣ��������۲�ʵ������ | ������ ��ɫ����������Ӵ���������ɺ���ɫ ��ɫ����������Ӵ���������ɺ���ɫ |
| 10-3a�qV +0.5 |
| 0.14 |
| 10-3a�qV +0.5 |
| 0.14 |
| 1 |
| 2 |
|
|
| 1 |
| 2 |
|
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�













 �Ƿ�Ϊͬ���칹��
�Ƿ�Ϊͬ���칹��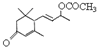 ��ͬ���칹����
��ͬ���칹����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ij����ֻ��C��H��O����Ԫ�أ������ģ����ͼ��ʾ�������й���12��ԭ�ӣ�ͼ��������֮���
ij����ֻ��C��H��O����Ԫ�أ������ģ����ͼ��ʾ�������й���12��ԭ�ӣ�ͼ��������֮���

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
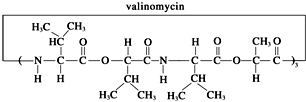
| A���Ӱ�ù�ز��ǵ����� | B���Ӱ�ù����ȫˮ��ɵõ����ְ����� | C���Ӱ�ù����ȫˮ���IJ����������ֲ��ﻥΪͬϵ�� | D���Ӱ�ù����ȫˮ�⣬����һ�ֲ�������ͻ�Ϊͬ���칹�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com