
ʵ���Һϳ����������ֲ�Ʒ�IJ������£�

��������ƿ�ڽ��������Ҵ�������ŨH2SO4��ϣ�Ȼ��Һ©���ߵμӴ��ᣬ��������ֱ���ռ������Ʒ�ɵõ������Ҵ������ѡ����ᡢ����ˮ�����������ֲ�Ʒ���ݴ˻ش����⣺
��1����Ӧ�м�����Ҵ��ǹ����ģ���Ŀ����
________________________________��
��2���ߵμӴ��ᣬ���������Ŀ����
_________________________________��
��3����ʵ����ʹ�����ιܳ������������⣬��һ��Ҫ������___________��
��4���Թ��б���̼������Һ��������___________________�� _______________��_____________________________��
��5��ʵ�����ɵ��������������ܶȱ�ˮ_______�������С��������_________ ��ζ��
��6��ʵ�������¶ȹ��ߣ���170�棩������������Ҫ�л�������__________�������ƣ���
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
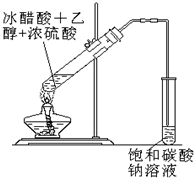 ʵ���Һϳ����������ֲ�Ʒ�ļ���װ�����ң��ɸ�ʵ����Եõ����������ֲ�Ʒ���ݴ���գ�
ʵ���Һϳ����������ֲ�Ʒ�ļ���װ�����ң��ɸ�ʵ����Եõ����������ֲ�Ʒ���ݴ���գ�| ���� |
| ���� |
| ���� |
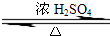 CH3COOCH2CH3+H2O��������Ӧ
CH3COOCH2CH3+H2O��������Ӧ| ���� |
| ���� |
| ���� |
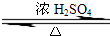 CH3COOCH2CH3+H2O��������Ӧ
CH3COOCH2CH3+H2O��������Ӧ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
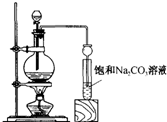 ʵ���Һϳ����������ֲ�Ʒ�IJ������£���������ƿ�ڽ��������Ҵ�������Ũ�����ϣ�Ȼ��Һ©���ߵμӴ����������ֱ���ռ������Ʒ�ɵõ������Ҵ������ѡ����ᡢ����ˮ�����������ֲ�Ʒ���ݴ˻ش����⣺
ʵ���Һϳ����������ֲ�Ʒ�IJ������£���������ƿ�ڽ��������Ҵ�������Ũ�����ϣ�Ȼ��Һ©���ߵμӴ����������ֱ���ռ������Ʒ�ɵõ������Ҵ������ѡ����ᡢ����ˮ�����������ֲ�Ʒ���ݴ˻ش����⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ���㽭ʡ����һ�и߶�10���¿���ѧ�Ծ����������� ���ͣ�ʵ����
��֪�Ҵ����Ժ��Ȼ��Ʒ�Ӧ��������ˮ��CaCl2��6C2H5OH���йص��л��Լ��ķе����£�CH3COOC2H5 77.1�� C2H5OC2H5(����) 34.5�� C2H5OH 78.3�� CH3COOH 118�� ʵ���Һϳ����������ֲ�Ʒ�IJ������£���������ƿ�ڽ��������Ҵ�������Ũ�����ϣ�Ȼ��Һ©���ߵμӴ��ᡢ�������õ������Ҵ������ѡ������ˮ�����������ֲ�Ʒ��
(1)��Ӧ�м����Ҵ��ǹ����ģ���Ŀ���ǣ� ��
���ֲ�Ʒ�پ����в��辫�ƣ�
(2)Ϊ��ȥ���еĴ��ᣬ�����Ʒ�м���__________������ĸ����
A ��ˮ�Ҵ� B ̼���Ʒ�ĩ C ��ˮ������
(3)�������м��뱥���Ȼ�����Һ������Ŀ���ǣ� ��
(4)Ȼ���������м�����ˮ�����ƣ�����Ŀ���ǣ� ��
������������������Һ�������һ���������ƿ�ڣ���������ȥ�ͷе���֣��ռ�76~78��г�֮�����ּ��á�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�������и߶���ѧ�����п��Ի�ѧ�Ծ� ���ͣ�ʵ����
(11��) ��֪�Ҵ����Ժ��Ȼ��Ʒ�Ӧ��������ˮ��CaCl2��6C2H5OH���йص��л��Լ��ķе����£�CH3COOC2H5 77.1�� C2H5OC2H5(����) 34.5�� C2H5OH 78.3�� CH3COOH 118�� ʵ���Һϳ����������ֲ�Ʒ�IJ������£���������ƿ�ڽ��������Ҵ�������Ũ�����ϣ�Ȼ��Һ©���ߵμӴ��ᡢ�������õ������Ҵ������ѡ������ˮ�����������ֲ�Ʒ��
��1��д���ϳ����������ķ�Ӧԭ����____________________________________________��
��2����Ӧ�м����Ҵ��ǹ����ģ���Ŀ���ǣ� ��
���ֲ�Ʒ�پ����в��辫�ƣ�
��3��Ϊ��ȥ�ֲ�Ʒ���еĴ��ᣬ�����Ʒ�м���__________������ĸ����
A����ˮ�Ҵ� B��̼���Ʒ�ĩ C����ˮ������
��4���������м��뱥���Ȼ�����Һ������Ŀ���ǣ� ��
��5��������������������Һ�������һ���������ƿ�ڣ�����������ʱ����Ҫ�IJ��������У��ƾ��ơ��¶ȼơ������ܡ���ƿ��β�ӹܺ�____________�������ܵ�ˮ������Ϊ �� ������ȥ�ͷе���֣��ռ�76��78��г�֮�����ּ��á�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com