



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 ŹµŃéŹŅĄļŅŖÅäÖĘ400mL0.2mol/LµÄĮņĖįÄĘČÜŅŗ£®ŹµŃé²½Öč“óÖĀÓŠ£ŗ
ŹµŃéŹŅĄļŅŖÅäÖĘ400mL0.2mol/LµÄĮņĖįÄĘČÜŅŗ£®ŹµŃé²½Öč“óÖĀÓŠ£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŹµŃéŹŅĄļŅŖÅäÖĘ400mL0.2mol/LµÄĮņĖįÄĘČÜŅŗ”£ŹµŃé²½Öč“óÖĀÓŠ£ŗ
A£®ŌŚĢģĘ½ÉĻ³Ę³ö___________gĮņĖįÄĘ¹ĢĢ壬°ŃĖü·ÅŌŚÉÕ±Ąļ£¬ÓĆŹŹĮæµÄÕōĮóĖ®Čܽā”£
B£®°ŃµĆµ½µÄČÜŅŗĄäČ“ŗ󊔊ĵŲŃŲ×Å__________×¢Čė________mLµÄČŻĮæĘæÖŠ”£
C£®ÓĆÉŁĮæÕōĮóĖ®Ļ“µÓÉÕ±ŗĶ²£Į§°ō2”«3“Ī£¬Ćæ“ĪĻ“µÓŅŗŅ²Š”ŠÄ×ŖČėČŻĮæĘæÖŠ”£
D£®¼ĢŠųĻņČŻĮæĘæÖŠ¼ÓÕōĮóĖ®ÖĮŅŗĆę¾ąæĢ¶Čl~2cm“¦£¬øÄÓĆ_______________Š”ŠÄµĪ¼ÓÕōĮóĖ®ÖĮČÜŅŗ°¼ŅŗĆęµ×²æÓėæĢ¶ČĻßĖ®Ę½ĻąĒŠ”£
E£®½«ĘæČūČū½ō£¬³ä·ÖŅ”ŌČ”£
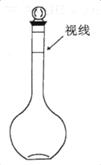 F£®½«ÅäŗƵÄČÜŅŗµ¹ČėŹŌ¼ĮĘæÖŠ£¬ĢłÉĻ±źĒ©£¬²¢Ļ“µÓČŻĮæĘ攣
F£®½«ÅäŗƵÄČÜŅŗµ¹ČėŹŌ¼ĮĘæÖŠ£¬ĢłÉĻ±źĒ©£¬²¢Ļ“µÓČŻĮæĘ攣
(1) ĒėĢīŠ“ÉĻŹöæհד¦”£
(2) ĻĀĮŠĒéæö»įŹ¹ĖłÅäČÜŅŗÅضČĘ«øߵďĒ___________(ĢīŠņŗÅ)”£
a£®Ä³Ķ¬Ń§¶ØČŻŹ±¹Ū²ģŅŗĆęµÄĒéæöČēĶ¼ĖłŹ¾
b£®Ć»ÓŠ½ųŠŠÉĻŹöµÄ²Ł×÷²½ÖčC
c£®Ņ”ŌČŗó·¢ĻÖŅŗĆęµĶÓŚæĢ¶ČĻßÓÖ¼ÓĖ®ĮĖ
d£®B²Ł×÷Ź±½«ÉŁĮæŅŗĢåČ÷µ½ĶāĆę
e£®ČŻĮæĘæÓĆĒ°ÄŚ±ŚÕ“ÓŠĖ®Öé
(3) Čē¹ūŹµŃéŹŅÓĆ18mol/LµÄÅØĮņĖįÅäÖĘ3. 6mol”¤L-1µÄĻ”ĮņĖį250mL”£¼ĘĖćĖłŠčÅØĮņĖįµÄĢå»żĪŖ___________mL£¬ŌŚŹµŃ鏱ӦÓĆ_________ĮæČ”ÅØĮņĖį”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğŗÓ±±Ź”ŹÆ¼Ņ×ÆŹŠÕż¶Ø֊ѧøßŅ»£ØÉĻ£©µŚŅ»“ĪŌĀæ¼»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗ½ā“šĢā
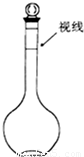
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010-2011ѧğĒąŗ£Ź”Ī÷ÄžŹŠ³Ē¶«ĒųĄ„ĀŲ֊ѧøßŅ»£ØÉĻ£©µŚŅ»“ĪŌĀæ¼»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com