
(1)1��![]() ���Ӻ���________�����ӣ�
���Ӻ���________�����ӣ�![]() �ĵ���ʽΪ________��
�ĵ���ʽΪ________��
(2)�γ�![]() ���ӵ����Է��ӵĽṹʽ��________��
���ӵ����Է��ӵĽṹʽ��________��
(3)д��![]() ������ǿ����Һ�з�Ӧ�����ӷ���ʽ________________________��
������ǿ����Һ�з�Ӧ�����ӷ���ʽ________________________��
(4)����A�Ļ�ѧʽΪNH5����������ԭ������㶼����ϡ������ԭ�ӵ��������ӽṹ���������ʵ����Ⱦͷֽ���������壬�Իش�
�ٹ���A����________���壬���ĵ���ʽ________��
��A����ˮ����Һ��________��(��ᡱ������С�)����ԭ���û�ѧ����ʽ��ʾΪ________________________��
��A���ȷֽ�Ļ�ѧ����ʽ��________________________��
| (1)22, (2) (3)N2H62- +2OH-====N2H4+2H2O (4)������, �ڼ�,NH4H+H2O====NH3��H2O+H2������NH5  
��ϰ��ϵ�д�
 ���Ͱ�ͨ��ĩ���ϵ�д� ���Ͱ�ͨ��ĩ���ϵ�д�
���ϰ��
��Ŀ�����л�ѧ ��Դ�� ���ͣ� ��Ԫ�ؿ��γɶ������ӣ���N3-��N3-��NH2-��NH4+��N2H5+��N2H62+�ȣ� ��1����N���ڵ�����Ԫ��C��O�����ߵ�һ�������ɴ�СΪ N��O��C N��O��C ��2��N��N�ļ���Ϊ942kJ?mol-1��N-N�����ļ���Ϊ247kJ?mol-1��˵��N2�е� �� �� ������ �� ���ȶ�����ҡ��С�������3��Һ̬���ɵ����NH2-��NH2-�ĵ���ʽΪ   ��4����֪NH4HΪ���ӻ����д��������ˮD2O��Ӧ����������ȣ� HD��NH3��HDO HD��NH3��HDO ��Na3NҲΪ���ӻ������Na3N��ˮ��Ӧ�Ļ�ѧ����ʽΪNa3N+3H2O=NH3��+3NaOH Na3N+3H2O=NH3��+3NaOH ����Ӧ����Ϊˮ�ⷴӦ ˮ�ⷴӦ ����5��X+�����е������ó���K��L��M�������Ӳ㣬����N3-�γɵľ���ṹ��ͼ1��ʾ����ͬһ��N3-������X+�� 6 6 ����Xԭ�ӵĻ�̬�����Ų�ʽΪ1s22s22p63s23p63d104S1 1s22s22p63s23p63d104S1 ��6�������ѧ�����Ƴ�ijԪ��Z��NԪ���γɵľ���ZN����֪ZN���������NaCl���Ƶľ���ṹ��ͼ2�Ǵ�ZN����ṹͼ�зָ�����IJ��ֽṹͼ�����жϷ���ZN����ṹͼ���� AD AD ��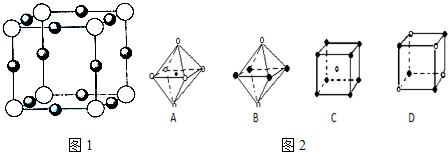 �鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ��2013�켪�ֳ���ʵ����ѧ�߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ������ ��9�֣���Ԫ�ؿ��γɶ������ӣ���N3����N3����NH2����NH4+��N2H5+��N2H62+�ȡ� ��1����N���ڵ�����Ԫ��C��O�����ߵ�һ�������ɴ�СΪ_______ ��2��N��N�ļ���Ϊ942 kJ��mol-1��N��N�����ļ���Ϊ247 kJ��mol-1��˵��N2�е� ���� ���ȶ�����ҡ��С����� ��3��Һ̬���ɵ����NH2����NH2���ĵ���ʽΪ �� ��4����֪NH4HΪ���ӻ����д��������ˮD2O��Ӧ����������ȣ� ��Na3NҲΪ���ӻ������Na3N��ˮ��Ӧ�Ļ�ѧ����ʽΪ ����Ӧ����Ϊ �� ��5��X+�����е������ó���K��L��M�������Ӳ㣬����N3���γɵľ���ṹ��ͼ��ʾ����ͬһ��N3��������X+ �� ����Xԭ�ӵĻ�̬�����Ų�ʽΪ_____________
��6�������ѧ�����Ƴ�ijԪ��Z��NԪ���γɵľ���ZN����֪ZN���������NaCl���Ƶľ���ṹ����ͼ�Ǵ�ZN����ṹͼ�зָ�����IJ��ֽṹͼ�����жϷ���ZN����ṹͼ����_______
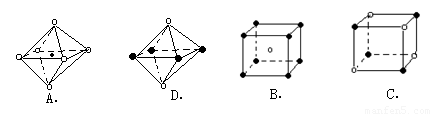
�鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�������и���9���¿������ۺ����⣨��ѧ���֣� ���ͣ������ (14��)���ǵ����ϼ�Ϊ�ḻ��Ԫ�أ���Ԫ�ؿ��γɶ������ӣ��磺N3����N3����NH2����NH4+��N2H5+��N2H62+�ȡ� (1)�뻭������ԭ�ӽṹʾ��ͼ___________�� (2)���ij����⻯��ĽṹʽΪ________����ռ乹��Ϊ_______������������ˮ����Ҫԭ����_________________������ˮ���Լ��Ե�ԭ����_______________(�����ӷ���ʽ˵��) (3)X+�����е������ó���K��L�������Ӳ㣬����N3���γɵľ���ṹ��ͼ26�� l��ʾ����ͬһ��N3��������X+��________����X��Ԫ�ط�����________��
(4)�����ѧ�����Ƴ�ijԪ��Z��NԪ���γɵľ���ZN����֪ZN���������NaCI���Ƶľ���ṹ��ͼ26��-2�Ǵ�ZN����ṹͼ�зָ�����IJ��ֽṹͼ�����жϷ���ZN����ṹͼ����_______________��
�鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ��0113 ������ ���ͣ������ ��Ԫ�ؿ��γɶ������ӣ���N3-��N3-��NH2-��NH4+��N2H5+��N2H62+�ȣ�����N2H62+ ��N2H5+�������Է��ӽ�������γɵģ���������NH4+�����ʡ��ش��������� ��1��N3-���Ӱ뾶��Na+���Ӱ뾶__________�����С���� ��2��д��NH4+������ǿ����Һ��Ӧ�����ӷ���ʽ______________________________ ��3��NH4+������H-N-H���ļ���Ϊ__________ ��4��д��Na3N��ˮ��Ӧ�Ļ�ѧ����ʽ______________________________ �鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ�� ���ͣ� (08�㽭��ɽ��ѧ�¿�)��Ԫ�ؿ��γɶ������ӣ���N3�D��N3�D��NH2�D��NH4+��N2H5+��N2H62+�ȣ�����N2H62+�������Է��ӽ�������γɵģ���������NH4+�����ʡ��ش��������⣺ ��1��N3�D���Ӱ뾶��Na+���Ӱ뾶 �����С���� ��2��д��Na3N��ˮ��Ӧ�Ļ�ѧ����ʽ ��3��NH4+������N�DH���ļ���Ϊ ��д�����ӻ�����NH5�ĵ���ʽ ��4��д��N2H62+���������ǿ����Һ��Ӧ�����ӷ���ʽ
�鿴�𰸺ͽ���>> ͬ����ϰ��� ����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר�� Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com��Ȩ��������վ�������£�ͼƬ��Դ�����磬����Ȩ����Ȩ��ԭ�������У�ת�������ַ���Ȩ��������Ȩ����������������֪�����ǽ����촦������ϵqq��3310059649�� ICP�������: ��ICP��07509807��-10 ����������42018502000812�� | |||||||||||||||||||||||