
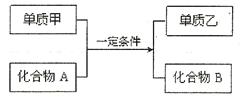
 Al2O3+2Fe(2��) (2)�����ά��2�֣�
Al2O3+2Fe(2��) (2)�����ά��2�֣� Al2O3+2Fe��
Al2O3+2Fe��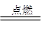 2MgO��C������CO2����������þ�ǻ�ԭ�������ߵ����ʵ���֮����1:2��2.4̼��0.2mol��������ҪCO2��0.2mol����״���µ�������4.48L��
2MgO��C������CO2����������þ�ǻ�ԭ�������ߵ����ʵ���֮����1:2��2.4̼��0.2mol��������ҪCO2��0.2mol����״���µ�������4.48L��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| ������ | Na+��Ba2+��NH4+ |
| ������ | CH3COO����Cl����OH����SO42�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
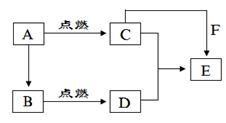
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
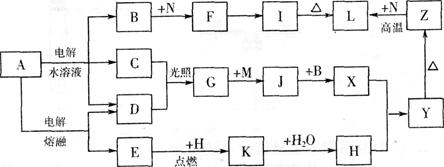
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
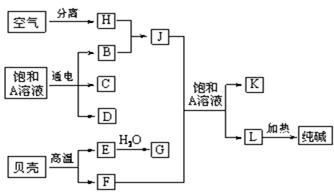
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
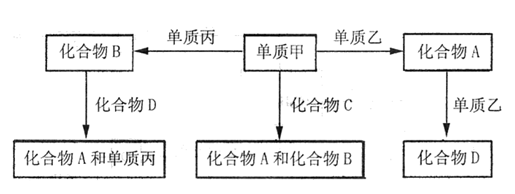
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ÿһ����Ԫ�ض��ǴӼ������ʼ����ϡ��������� |
| B��f�����Ǹ���Ԫ�أ�s����p���Ķ�������Ԫ�� |
| C����֪��200C 1mol Naʧȥ1 mol����������650kJ�����������һ������Ϊ650KJ/mol�� |
| D��Ge�ĵ縺��Ϊ1.8�������ǵ��͵ķǽ��� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com