
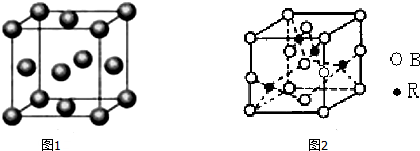
���� ��1���������Ӽ��ľ��������Ӿ��壬���Ӿ����п��ܺ��й��ۼ�������ͨ�����Ӽ���������ϵ����ڷ��Ӿ��壻
��2��������ֻ����һ��������������˵��������Ϊԭ�Ӿ����ԭ�ӷ��ӵķ��Ӿ����ֻ�����Ӽ������Ӿ����������壻
��3��ԭ�Ӿ����ۻ�ʱ�ƻ����ۼ���
��4���������Ӽ��ľ��������Ӿ��壬���Ӿ����п��ܺ��й��ۼ���
��5�����Ӿ�����岻���磬��ѹ���ۻ�ʱ�ܵ��磮
��� �⣺��CaBr2 �������Ӿ��壬ֻ�������Ӽ���
�ڶ��������д��ڹ��ۼ�������ԭ�Ӿ��壻
��Ar������û�й��ۼ���ֻ�з��Ӽ������������ڷ��Ӿ��壻
��ͭ �ǽ������壬������ֻ����һ�������������ǽ�������
�ݣ�NH4��2SO4�����мȺ����Ӽ����ֺ����ۼ���
�ɱ����Ӵ��ڹ��ۼ��ͷ��Ӽ������������ڷ��Ӿ��壻
�߳����µĴ�������ڹ��ۼ��ͷ��Ӽ������������ڷ��Ӿ��壻
����״��ڹ��ۼ��ͷ��Ӽ������������ڷ��Ӿ��壻
��1���������Ӽ��ľ��������Ӿ��壬���Ӿ����п��ܺ��й��ۼ����������Ӿ�����Ǣ٢ݣ�����ͨ�����Ӽ���������ϵ����ڷ��Ӿ��壬��ۢޢߢ����ڷ��Ӿ��壻
�ʴ�Ϊ���٢ݣ��ۢޢߢࣻ
��2��������ֻ����һ��������������˵��������Ϊԭ�Ӿ����ԭ�ӷ��ӵķ��Ӿ����ֻ�����Ӽ������Ӿ����������壬��Ϊ�٢ڢܣ��ʴ�Ϊ���٢ڢܣ�
��3��ԭ�Ӿ����ۻ�ʱ�ƻ����ۼ�������ԭ�Ӿ����Ϊ���ڣ��ʴ�Ϊ���ڣ�
��4���������Ӽ��ľ��������Ӿ��壬���Ӿ����п��ܺ��й��ۼ����Ⱥ����Ӽ����ֺ����ۼ����Ǣݣ��ʴ�Ϊ���ݣ�
��5�����Ӿ�����岻���磬��ѹ���ۻ�ʱ�ܵ��磬���Թ��岻���磬��ѹ���ۻ�ʱ�ܵ�����Ǣ٢ݣ��ʴ�Ϊ���٢ݣ�
���� ���⿼���˾����д��ڵ������������ݾ���Ĺ�����������������������������Ŀ�ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | SiO2 CaO NaCl CBr4 CF4 | |
| B�� | SiO2 NaCl CaO CF4 CBr4 | |
| C�� | CaO NaCl SiO2 CBr4 CF4 | |
| D�� | CF4CBr4NaCl CaO SiO2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ɽ�廬�� | B�� | ������ | C�� | �����ˮ | D�� | �ⵥ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���� | B�� | ���� | C�� | �ᾧ | D�� | ��ȡ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
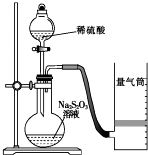 ��ѧ��Ӧ������������ѧ��Ӧ���п����̶ȵ���������������ijͬѧ�ⶨ��ѧ��Ӧ���ʲ�̽����Ӱ�����ص�ʵ�飮
��ѧ��Ӧ������������ѧ��Ӧ���п����̶ȵ���������������ijͬѧ�ⶨ��ѧ��Ӧ���ʲ�̽����Ӱ�����ص�ʵ�飮| ʵ����� | ���V/mL | ʱ��/s | |||
| Na2S2O3��Һ | ������Һ | ��ˮ | ˮ | ||
| �� | 10.0 | 2.0 | 4.0 | 0.0 | t1 |
| �� | 8.0 | 2.0 | 4.0 | 2.0 | t2 |
| �� | 6.0 | 2.0 | 4.0 | Vx | t3 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com