

| ¶ĮŹż“ĪŹż | ÖŹĮæ£Øg£© |
׶ŠĪĘæ+Ė®+ŹŌŃł | µŚŅ»“Ī | 192.214 |
µŚ¶ž“Ī | 192.164 | |
µŚČż“Ī | 192.028 | |
µŚĖÄ“Ī | 192.010 | |
µŚĪå“Ī | 192.010 |
(1)Š“³öNa2O2ŗĶH2O·“Ó¦µÄ»Æѧ·½³ĢŹ½ ”£
(2)¼ĘĖć¹żŃõ»ÆÄʵÄÖŹĮæ·ÖŹżŹ±£¬±ŲŠčµÄŹż¾ŻŹĒ £¬²»±Ų×÷µŚĮł“Ī¶ĮŹżµÄŌŅņŹĒ ”£
(3)²ā¶ØÉĻŹöѳʷ£Ø
![]()
¢Ł²Ł×÷¢ņµÄĆū³ĘŹĒ ”£
¢ŚŠčÖ±½Ó²ā¶ØµÄĪļĄķĮæŹĒ ”£
¢Ū²ā¶Ø¹ż³ĢÖŠŠčŅŖµÄŅĒĘ÷ÓŠµē×ÓĢģĘ½”¢Õō·¢Ćó”¢¾Ę¾«µĘ£¬»¹ŠčŅŖ ”¢ £Ø¹Ģ¶Ø”¢¼Š³ÖŅĒĘ÷³żĶā£©”£
¢ÜŌŚ×ŖŅĘČÜŅŗŹ±£¬ČēČÜŅŗ×ŖŅĘ²»ĶźČ«£¬ŌņNa2O2µÄÖŹĮæ·ÖŹżµÄ²ā¶Ø½į¹ū £ØĢī”°Ę«“ó”±”°Ę«Š””±»ņ”°²»±ä”±£©”£
½āĪö£ŗ£Ø1£©2Na2O2+2H2O![]() 4NaOH+O2”ü
4NaOH+O2ӟ
£Ø2£©w(Na2O2)= ![]() ”Į100%£¬¼ĘĖćw(Na2O2)ĖłŠčµÄm(ŹŌŃł)ŅŃÖŖ£¬¶ųĖłŠčµÄm(Na2O2)ŠčŅŖ½čÖś·“Ó¦µÄ»Æѧ·½³ĢŹ½ŗĶŅĒĘ÷²āĮæµÄŹż¾Ż¼ĘĖć³öĄ“”£ŹµŃé¹ż³ĢÖŠµē×ÓĢģĘ½ĻŌŹ¾µÄ×ÜÖŹĮæ²»¶Ļ¼õŠ”£¬ÕāŹĒÓÉ·“Ó¦²śÉśµÄO2ŅŻ³ö¶ųŅżĘšµÄ”£Na2O2ĶźČ«·“Ó¦Ź±£¬²śÉśO2µÄÖŹĮæµČÓŚŹŌŃł”¢×¶ŠĪĘæŗĶĖ®µÄ×ÜÖŹĮæ¼õČ„·“Ó¦ÖÕÖ¹Ź±×¶ŠĪĘæŗĶ·“Ó¦»ģŗĻŅŗµÄ×ÜÖŹĮæ£Øµē×ÓĢģĘ½µŚĖÄ“Ī»ņµŚĪå“ĪĻŌŹ¾µÄÖŹĮ棩”£½čÖś·“Ó¦µÄ·½³ĢŹ½ŗĶ²śÉśO2µÄÖŹĮ漓æɼĘĖć³öĖłŠčµÄŹż¾Żm(Na2O2)”£×ŪŗĻŅŌÉĻ·ÖĪö£¬¼ĘĖćNa2O2µÄÖŹĮæ·ÖŹżŹ±£¬ŠčŅŖŹŌŃłÖŹĮ攢׶ŠĪĘæŗĶĖ®µÄÖŹĮ攢µŚĖģػņĪ壩“Ī¶ĮŹż£Ø»ņÓĆ¾ßĢåŹżÖµ±ķŹ¾£©£¬¹²ČżøöŹż¾Ż”£²»±Ų×÷µŚĮł“Ī¶ĮŹżµÄŌŅņŹĒµŚĖÄŗĶµŚĪå“Ī¶ĮŹżŹ±×¶ŠĪĘæÄŚÖŹĮæŅŃ“ļŗćÖŲ”££Ø3£©ŹŌŃłÖŠ¼ÓČėŃĪĖįŹ±·¢ÉśĻĀĮŠ·“Ó¦£ŗNa2O2+2HCl
”Į100%£¬¼ĘĖćw(Na2O2)ĖłŠčµÄm(ŹŌŃł)ŅŃÖŖ£¬¶ųĖłŠčµÄm(Na2O2)ŠčŅŖ½čÖś·“Ó¦µÄ»Æѧ·½³ĢŹ½ŗĶŅĒĘ÷²āĮæµÄŹż¾Ż¼ĘĖć³öĄ“”£ŹµŃé¹ż³ĢÖŠµē×ÓĢģĘ½ĻŌŹ¾µÄ×ÜÖŹĮæ²»¶Ļ¼õŠ”£¬ÕāŹĒÓÉ·“Ó¦²śÉśµÄO2ŅŻ³ö¶ųŅżĘšµÄ”£Na2O2ĶźČ«·“Ó¦Ź±£¬²śÉśO2µÄÖŹĮæµČÓŚŹŌŃł”¢×¶ŠĪĘæŗĶĖ®µÄ×ÜÖŹĮæ¼õČ„·“Ó¦ÖÕÖ¹Ź±×¶ŠĪĘæŗĶ·“Ó¦»ģŗĻŅŗµÄ×ÜÖŹĮæ£Øµē×ÓĢģĘ½µŚĖÄ“Ī»ņµŚĪå“ĪĻŌŹ¾µÄÖŹĮ棩”£½čÖś·“Ó¦µÄ·½³ĢŹ½ŗĶ²śÉśO2µÄÖŹĮ漓æɼĘĖć³öĖłŠčµÄŹż¾Żm(Na2O2)”£×ŪŗĻŅŌÉĻ·ÖĪö£¬¼ĘĖćNa2O2µÄÖŹĮæ·ÖŹżŹ±£¬ŠčŅŖŹŌŃłÖŹĮ攢׶ŠĪĘæŗĶĖ®µÄÖŹĮ攢µŚĖģػņĪ壩“Ī¶ĮŹż£Ø»ņÓĆ¾ßĢåŹżÖµ±ķŹ¾£©£¬¹²ČżøöŹż¾Ż”£²»±Ų×÷µŚĮł“Ī¶ĮŹżµÄŌŅņŹĒµŚĖÄŗĶµŚĪå“Ī¶ĮŹżŹ±×¶ŠĪĘæÄŚÖŹĮæŅŃ“ļŗćÖŲ”££Ø3£©ŹŌŃłÖŠ¼ÓČėŃĪĖįŹ±·¢ÉśĻĀĮŠ·“Ó¦£ŗNa2O2+2HCl![]() 2NaCl+H2O2£¬Na2O+2HCl
2NaCl+H2O2£¬Na2O+2HCl![]() 2NaCl+H2O£¬ÕŅ²»µ½ÕāĮ½øö·“Ó¦Ö®ŗóÓŠ¼ĘĖć¼ŪÖµµÄæɲāŹż¾Ż£¬±ŲŠėŌŁ¼ÓČČŹ¹H2O2·Ö½ā£¬²¢Ź¹Ź£ÓąµÄHClŗĶH2O»Ó·¢µō£¬²ÅÄܳĘĮæNaClµÄÖŹĮæÕāŅ»ÓŠ¼ĘĖć¼ŪÖµµÄŹż¾Ż”£ŅĄ¾ŻÕāŅ»²āĮæĖ¼Ā·£¬æÉŅŌČ·¶ØŹµŃé²Ł×÷Į÷³ĢÖŠµÄ²Ł×÷¢ńŹĒŌŚ¼ÓČČĢõ¼žĻĀ·“Ó¦£¬²Ł×÷¢ņŹĒ¼ÓČČÕōøÉČÜŅŗ£¬»¹ŠčŅŖÖ±½Ó²ā¶ØµÄĪļĄķĮæŹĒÕōøÉŗóµÄNaCl¹ĢĢåµÄÖŹĮ棬»¹ŠčŅŖµÄŅĒĘ÷ŹĒÉÕ±”¢²£Į§°ō”£¼ĘĖ揱£¬ÉčNa2O2”¢Na2OµÄÖŹĮæ·Ö±šĪŖm1”¢m2£¬ĄūÓĆĖł²āŹż¾ŻæÉĮŠ³öČēĻĀĮ½øö·½³ĢŹ½£ŗm1+m2=
2NaCl+H2O£¬ÕŅ²»µ½ÕāĮ½øö·“Ó¦Ö®ŗóÓŠ¼ĘĖć¼ŪÖµµÄæɲāŹż¾Ż£¬±ŲŠėŌŁ¼ÓČČŹ¹H2O2·Ö½ā£¬²¢Ź¹Ź£ÓąµÄHClŗĶH2O»Ó·¢µō£¬²ÅÄܳĘĮæNaClµÄÖŹĮæÕāŅ»ÓŠ¼ĘĖć¼ŪÖµµÄŹż¾Ż”£ŅĄ¾ŻÕāŅ»²āĮæĖ¼Ā·£¬æÉŅŌČ·¶ØŹµŃé²Ł×÷Į÷³ĢÖŠµÄ²Ł×÷¢ńŹĒŌŚ¼ÓČČĢõ¼žĻĀ·“Ó¦£¬²Ł×÷¢ņŹĒ¼ÓČČÕōøÉČÜŅŗ£¬»¹ŠčŅŖÖ±½Ó²ā¶ØµÄĪļĄķĮæŹĒÕōøÉŗóµÄNaCl¹ĢĢåµÄÖŹĮ棬»¹ŠčŅŖµÄŅĒĘ÷ŹĒÉÕ±”¢²£Į§°ō”£¼ĘĖ揱£¬ÉčNa2O2”¢Na2OµÄÖŹĮæ·Ö±šĪŖm1”¢m2£¬ĄūÓĆĖł²āŹż¾ŻæÉĮŠ³öČēĻĀĮ½øö·½³ĢŹ½£ŗm1+m2=![]() ”Į2+
”Į2+![]() ”Į2)”Į
”Į2)”Į
“š°ø£ŗ£Ø1£©2Na2O2+2H2O![]() 4NaOH+O2”ü
4NaOH+O2ӟ
£Ø2£©ŹŌŃłÖŹĮ攢׶ŠĪĘæŗĶĖ®µÄÖŹĮ攢µŚĖģػņĪ壩“Ī¶ĮŹż£Ø»ņÓĆ¾ßĢåŹżÖµ±ķŹ¾£© ׶ŠĪĘæÄŚÖŹĮæŅŃ“ļŗćÖŲ
£Ø3£©¢ŁÕō·¢
¢ŚNaClµÄÖŹĮæ
¢ŪÉÕ±²£Į§°ō
¢ÜĘ«“ó


 ¶į¹Ś½š¾ķČ«ÄÜĮ·æ¼ĻµĮŠ“š°ø
¶į¹Ś½š¾ķČ«ÄÜĮ·æ¼ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
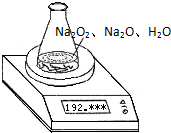 ijÖÖŗ¬ÓŠÉŁĮæŃõ»ÆÄĘµÄ¹żŃõ»ÆÄĘŹŌŃłÖŹĮæĪŖ1.56g£¬ĪŖ²ā¶Øø÷³É·ÖµÄÖŹĮæ·ÖŹż£¬°“ČēĶ¼ĖłŹ¾£¬Ō׶ŠĪĘæŗĶĖ®µÄ×ÜÖŹĮæĪŖ190.72£¬½«1.56gÉĻŹöѳʷĶ¶Čė׶ŠĪĘæÖŠ£¬³ä·Ö·“Ó¦ŗ󣬵ē×ÓĢģĘ½×īÖյĶĮŹżĪŖ192.12g£®
ijÖÖŗ¬ÓŠÉŁĮæŃõ»ÆÄĘµÄ¹żŃõ»ÆÄĘŹŌŃłÖŹĮæĪŖ1.56g£¬ĪŖ²ā¶Øø÷³É·ÖµÄÖŹĮæ·ÖŹż£¬°“ČēĶ¼ĖłŹ¾£¬Ō׶ŠĪĘæŗĶĖ®µÄ×ÜÖŹĮæĪŖ190.72£¬½«1.56gÉĻŹöѳʷĶ¶Čė׶ŠĪĘæÖŠ£¬³ä·Ö·“Ó¦ŗ󣬵ē×ÓĢģĘ½×īÖյĶĮŹżĪŖ192.12g£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
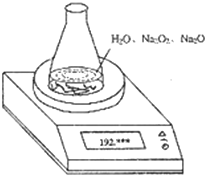 £Ø2004?ÉĻŗ££©Ä³ÖÖŗ¬ÓŠÉŁĮæŃõ»ÆÄĘµÄ¹żŃõ»ÆÄĘŹŌŃł£ØŅŃÖŖŹŌŃłÖŹĮæĪŖ1.560g”¢×¶ŠĪĘæŗĶĖ®µÄÖŹĮæĪŖ190.720g£©£¬ĄūÓĆČēĶ¼×°ÖĆ²ā¶Ø»ģŗĻĪļÖŠNa2O2µÄÖŹĮæ·ÖŹż£¬ĆæøōĻąĶ¬Ź±¼ä¶ĮµĆµē×ÓĢģĘ½µÄŹż¾ŻČē±ķ£ŗ
£Ø2004?ÉĻŗ££©Ä³ÖÖŗ¬ÓŠÉŁĮæŃõ»ÆÄĘµÄ¹żŃõ»ÆÄĘŹŌŃł£ØŅŃÖŖŹŌŃłÖŹĮæĪŖ1.560g”¢×¶ŠĪĘæŗĶĖ®µÄÖŹĮæĪŖ190.720g£©£¬ĄūÓĆČēĶ¼×°ÖĆ²ā¶Ø»ģŗĻĪļÖŠNa2O2µÄÖŹĮæ·ÖŹż£¬ĆæøōĻąĶ¬Ź±¼ä¶ĮµĆµē×ÓĢģĘ½µÄŹż¾ŻČē±ķ£ŗ| ¶ĮŹż“ĪŹż | ÖŹĮæ£Øg£© | |
| ׶ŠĪĘæ+Ė®+ŹŌŃł | µŚ1“Ī | 192.214 |
| µŚ2“Ī | 192.164 | |
| µŚ3“Ī | 192.028 | |
| µŚ4“Ī | 192.010 | |
| µŚ5“Ī | 192.010 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| ¶ĮŹż“ĪŹż | ÖŹĮæ/g |
׶ŠĪĘæ+Ė®+ŹŌŃł | µŚ1“Ī | 192.214 |
µŚ2“Ī | 192.164 | |
µŚ3“Ī | 192.028 | |
µŚ4“Ī | 192.010 | |
µŚ5“Ī | 192.010 |
£Ø1£©Š“³öNa2O2ŗĶH2O·“Ó¦µÄ»Æѧ·½³ĢŹ½________________________________”£
£Ø2£©¼ĘĖć¹żŃõ»ÆÄĘÖŹĮæ·ÖŹżŹ±£¬±ŲŠčµÄŹż¾ŻŹĒ________________”£²»±Ų×÷µŚ6“Ī¶ĮŹżµÄŌŅņŹĒ________________________________”£
£Ø3£©²ā¶ØÉĻŹöѳʷ£Ø1.560 g£©ÖŠNa2O2ÖŹĮæ·ÖŹżµÄĮķŅ»ÖÖ·½°ø£¬Ęä²Ł×÷Į÷³ĢČēĻĀ£ŗ
ѳʷ”żĻ”ŃĪĖį
![]()
¢Ł²Ł×÷¢ņµÄĆū³ĘŹĒ____________”£
¢ŚŠčÖ±½Ó²ā¶ØµÄĪļĄķĮæŹĒ____________”£
¢Ū²ā¶Ø¹ż³ĢÖŠŠčŅŖµÄŅĒĘ÷ÓŠµē×ÓĢģĘ½”¢Õō·¢Ćó”¢¾Ę¾«µĘ£¬»¹ŠčŅŖ________”¢________£Ø¹Ģ¶Ø”¢¼Š³ÖŅĒĘ÷³żĶā£©”£
¢ÜŌŚ×ŖŅĘČÜŅŗŹ±£¬ČēČÜŅŗ×ŖŅĘ²»ĶźČ«£¬ŌņNa2O2ÖŹĮæ·ÖŹżµÄ²ā¶Ø½į¹ū____________£ØĢī”°Ę«“ó”±”°Ę«Š””±»ņ”°²»±ä”±£©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010-2011ѧğĮÉÄžŹ”øßČżµŚĖÄ“ĪÄ£Äāæ¼ŹŌ£ØĄķ×Ū£©»Æѧ²æ·Ö ĢāŠĶ£ŗŹµŃéĢā
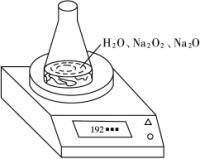
²āijÖÖŗ¬ÓŠÉŁĮæŃõ»ÆÄĘµÄ¹żŃõ»ÆÄĘŹŌŃłµÄÖŹĮæ·ÖŹż”£
·½·ØŅ»£ŗĄūÓĆĻĀĶ¼×°ÖĆ²ā¶Ø»ģŗĻĪļÖŠNa2O2µÄÖŹĮæ·ÖŹż£¬ŅŃÖŖŹŌŃłÖŹĮæĪŖ1.560g”¢×¶ŠĪĘæŗĶĖ®µÄÖŹĮæĪŖ190.720g£¬ĆæøōĻąĶ¬Ź±¼ä¶ĮµĆµē×ÓĢģĘ½µÄŹż¾ŻČē±ķ£ŗ

£Ø1£©Š“³öNa2O2ŗĶH2O·“Ó¦µÄ»Æѧ·½³ĢŹ½________________
£Ø2£©øĆŹŌŃłÖŠ¹żŃõ»ÆÄʵÄÖŹĮæ·ÖŹżĪŖ____________________£Ø±£Įō3Ī»ÓŠŠ§Źż×Ö£©
²»±Ų×÷µŚ6“Ī¶ĮŹżµÄŌŅņŹĒ____________________________________
·½·Ø¶ž£ŗ²ā¶ØÉĻŹöѳʷ£Ø1.560g£©ÖŠNa2O2ÖŹĮæ·ÖŹżµÄĮķŅ»ÖÖ·½°ø£¬Ęä²Ł×÷Į÷³ĢČēĻĀ£ŗ

£Ø3£©²Ł×÷¢ņµÄĆū³ĘŹĒ________________________
£Ø4£©ŠčÖ±½Ó²ā¶ØµÄĪļĄķĮæŹĒ______________________________
£Ø5£©²ā¶Ø¹ż³ĢÖŠŠčŅŖµÄŅĒĘ÷ÓŠµē×ÓĢģĘ½”¢Õō·¢Ćó”¢¾Ę¾«µĘ”¢»¹ŠčŅŖ________”¢_______£Ø¹Ģ¶Ø”¢¼Š³ÖŅĒĘ÷³żĶā£©
£Ø6£©ŌŚ×ŖŅĘČÜŅŗŹ±£¬ČēČÜŅŗ×ŖŅĘ²»ĶźČ«£¬ŌņNa2O2ÖŹĮæ·ÖŹżµÄ²ā¶Ø½į¹ū__________________
£ØĢīĘ«“ó”¢Ę«Š”»ņ²»±ä£©”£
·½·ØČż£ŗĒė“ÓĻĀĶ¼ÖŠŃ”ÓĆŹŹµ±ŅĒĘ÷²ā¶Ø»ģŗĻĪļÖŠNa2O2µÄÖŹĮæ·ÖŹż£¬ŅŖĒó²Ł×÷¼ņµ„”£
³ż“ż²āŹŌŃłĶā£¬ĻŽŃ”ŹŌ¼Į£ŗCaCO3¹ĢĢå,6mol/LŃĪĖįŗĶÕōĮóĖ®
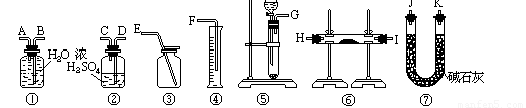
£Ø7£©ĖłŃ”ÓĆ×°ÖƵÄĮ¬½ÓĖ³ŠņÓ¦ŹĒ(Ģīø÷½ÓæŚµÄ×ÖÄø;Į¬½Ó½ŗ¹ÜŹ”ĀŌ)__________________”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com