
����12�֣����������л��ų������ı���[��Ҫ����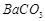 ��
��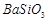 ��
��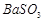 ��
��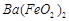 ��]��ij��������Ҫ����
��]��ij��������Ҫ����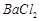 ��
��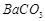 ��
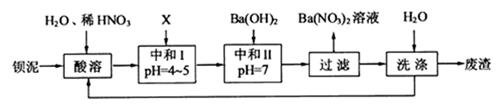
��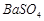 �������ñ�����ȡ
�������ñ�����ȡ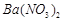 ���䲿�ֹ����������£�����֪����1��FeO2-����ˮ�������Fe(OH)3
(2)Fe3+��pH��3.7ʱ����ˮ�⼴������ȫ��
���䲿�ֹ����������£�����֪����1��FeO2-����ˮ�������Fe(OH)3
(2)Fe3+��pH��3.7ʱ����ˮ�⼴������ȫ��
��1�����ܺ���Һ��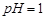 ��
��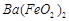 ��
��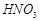 �ķ�Ӧ��ѧ����ʽΪ��
�ķ�Ӧ��ѧ����ʽΪ��
��
��2������ʱͨ�����Ʒ�Ӧ�¶Ȳ�����70�棬�Ҳ�ʹ��Ũ���ᣬ��ԭ���Ƿ�ֹ��Ӧ���ʹ��졢Ũ����ӷ����� ��
��3���ó���ϱ�������ʵ��ѡ�õ�XΪ ���ѧʽ�������к͢�ʹ��Һ�У������ӷ��ţ� ��Ũ�ȼ��١�
��4����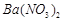 ��Һ�еõ��侧��ķ��뷽����
��
��Һ�еõ��侧��ķ��뷽����
��
��5��������ֱ���ŷš�����������ϴ�ӵ�Ŀ���� ��
��1��Ba(FeO2)2+8HNO3=Ba(NO3)2+2Fe(NO3)3+4H2O
��2���ֽ�
��3��BaCO3 Fe3+ H+
��4�������ᾧ
��5����ȥ�����еĿ����Ա��Σ���С�Ի�������Ⱦ
����������1���������֪FeO2������ˮ�������Fe(OH)3���������б�Ȼ������������
��2���������Ȳ��ȶ��ᣬ�ֽ�
��3��ʹ�ù������е�BaCO3����ʡԭ�ϣ�����H+�����pH��ʹFe3+ȫ��ת��Ϊ����
��5�����������ؽ����Σ���Ȼϴ�ӳ�ȥ����ֹ��Ⱦ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��16�֣����������л��ų������ı���[��Ҫ����![]() ��
��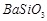 ��
��![]() ��
��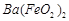 ��]��ij��������Ҫ����
��]��ij��������Ҫ����![]() ��
��![]() ��
��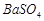 �������ñ�����ȡ
�������ñ�����ȡ![]() ���䲿�ֹ����������£�
���䲿�ֹ����������£�
��1�����ܺ���Һ��![]() ��
��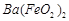 ��
��![]() �ķ�Ӧ��ѧ����ʽΪ ��
�ķ�Ӧ��ѧ����ʽΪ ��
��2������ʱͨ�����Ʒ�Ӧ�¶Ȳ�����70�棬�Ҳ�ʹ��Ũ���ᣬ��ԭ����
�� ��
��3���ó���ϱ�������ʵ��ѡ�õ�XΪ ���ѧʽ�������к͢�ʹ��Һ�� �������ӷ��ţ���Ũ�ȼ��٣����к͢��������Һ����仯�ɺ��ԣ���
��4������������ϴ�ӵ�Ŀ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013���������ʮ���и�����ѧ�ڵڶ����¿���ѧ�Ծ����������� ���ͣ�ʵ����
����12�֣����������л��ų������ı���[��Ҫ����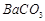 ��
��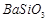 ��
��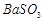 ��
��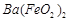 ��]��ij��������Ҫ����
��]��ij��������Ҫ����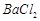 ��
��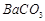 ��
��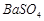 �������ñ�����ȡ
�������ñ�����ȡ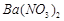 ���䲿�ֹ����������£�����֪����1��FeO2-����ˮ�������Fe(OH)3 (2)Fe3+��pH��3.7ʱ����ˮ�⼴������ȫ��
���䲿�ֹ����������£�����֪����1��FeO2-����ˮ�������Fe(OH)3 (2)Fe3+��pH��3.7ʱ����ˮ�⼴������ȫ��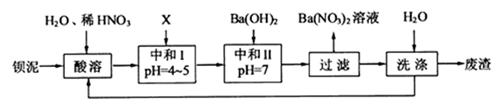
��1�����ܺ���Һ��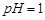 ��
��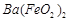 ��
��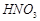 �ķ�Ӧ��ѧ����ʽΪ��
�ķ�Ӧ��ѧ����ʽΪ��
��
��2������ʱͨ�����Ʒ�Ӧ�¶Ȳ�����70�棬�Ҳ�ʹ��Ũ���ᣬ��ԭ���Ƿ�ֹ��Ӧ���ʹ��졢Ũ����ӷ����� ��
��3���ó���ϱ�������ʵ��ѡ�õ�XΪ ���ѧʽ�������к͢�ʹ��Һ�У������ӷ��ţ� ��Ũ�ȼ��١�
��4����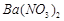 ��Һ�еõ��侧��ķ��뷽���� ��
��Һ�еõ��侧��ķ��뷽���� ��
��5��������ֱ���ŷš�����������ϴ�ӵ�Ŀ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011��㶫ʡ����ȫ��߿�ģ���Ծ���һ�������ۣ���ѧ���� ���ͣ������
��16�֣����������л��ų������ı���[��Ҫ����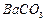 ��
��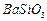 ��
��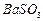 ��
��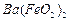 ��]��ij��������Ҫ����
��]��ij��������Ҫ����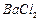 ��
��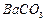 ��
��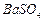 �������ñ�����ȡ
�������ñ�����ȡ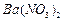 ���䲿�ֹ����������£�
���䲿�ֹ����������£�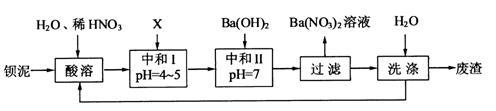
��1�����ܺ���Һ��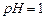 ��
��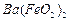 ��
��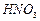 �ķ�Ӧ��ѧ����ʽΪ ��
�ķ�Ӧ��ѧ����ʽΪ ��
��2������ʱͨ�����Ʒ�Ӧ�¶Ȳ�����70�棬�Ҳ�ʹ��Ũ���ᣬ��ԭ����
�� ��
��3���ó���ϱ�������ʵ��ѡ�õ�XΪ ���ѧʽ�������к͢�ʹ��Һ�� �������ӷ��ţ���Ũ�ȼ��٣����к͢��������Һ����仯�ɺ��ԣ���
��4������������ϴ�ӵ�Ŀ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ��㶫ʡ����ȫ��߿�ģ���Ծ���һ�������ۣ���ѧ���� ���ͣ������
��16�֣����������л��ų������ı���[��Ҫ���� ��
�� ��
�� ��
��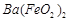 ��]��ij��������Ҫ����
��]��ij��������Ҫ���� ��
�� ��
��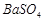 �������ñ�����ȡ
�������ñ�����ȡ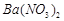 ���䲿�ֹ����������£�
���䲿�ֹ����������£�
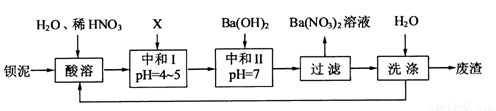
��1�����ܺ���Һ�� ��
��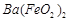 ��
�� �ķ�Ӧ��ѧ����ʽΪ
��
�ķ�Ӧ��ѧ����ʽΪ
��
��2������ʱͨ�����Ʒ�Ӧ�¶Ȳ�����70�棬�Ҳ�ʹ��Ũ���ᣬ��ԭ����
�� ��
��3���ó���ϱ�������ʵ��ѡ�õ�XΪ ���ѧʽ�������к͢�ʹ��Һ�� �������ӷ��ţ���Ũ�ȼ��٣����к͢��������Һ����仯�ɺ��ԣ���
��4������������ϴ�ӵ�Ŀ���� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com