
����Ŀ��A��B��C��D�����ֶ�����Ԫ�أ�E�ǹ���Ԫ�ء�A��B��Cͬ���ڣ�C��Dͬ���壬A��ԭ�ӽṹʾ��ͼΪ ��B��ͬ���ڵ�һ��������С��Ԫ�أ�C�������������δ�ɶԵ��ӣ�E����Χ�����Ų�ʽΪ3d64s2���ش��������⣺
��B��ͬ���ڵ�һ��������С��Ԫ�أ�C�������������δ�ɶԵ��ӣ�E����Χ�����Ų�ʽΪ3d64s2���ش��������⣺
��1��д������Ԫ�صķ��ţ�A________��B________��C________��D________��
��2���û�ѧʽ��ʾ��������Ԫ��������������Ӧˮ����������ǿ����________��������ǿ����__________��
��3����Ԫ�ط��ű�ʾD�������ڵ�һ����������Ԫ����________���縺�ԣ���ϡ�������⣩����Ԫ����_________��
��4��EԪ��ԭ�ӵĺ˵������_________��EԪ�������ڱ��ĵ�_______���ڵ�_______�壬��________����
��5��д��DԪ��ԭ�ӹ��ɵ��ʵĵ���ʽ___________���÷�������_______���Ҽ���_______���м���
���𰸡�Si Na P N HNO3 NaOH Ne F 26 4 VIII d ![]() 1 2
1 2
��������
A��B��C��D�����ֶ�����Ԫ�أ�E�ǹ���Ԫ�ء�A��B��Cͬ���ڣ�C��Dͬ���壬A��ԭ�ӽṹʾ��ͼΪ ����x=2��AΪ14��Ԫ��Si��B��ͬ���ڵ�һ��������С��Ԫ�أ���BΪNaԪ�أ�C�������������δ�ɶԵ��ӣ���CΪPԪ�ء�DΪNԪ�أ�E����Χ�����Ų�ʽΪ3d64s2����EΪ26��Ԫ��Fe��
����x=2��AΪ14��Ԫ��Si��B��ͬ���ڵ�һ��������С��Ԫ�أ���BΪNaԪ�أ�C�������������δ�ɶԵ��ӣ���CΪPԪ�ء�DΪNԪ�أ�E����Χ�����Ų�ʽΪ3d64s2����EΪ26��Ԫ��Fe��
��1��A��B��C��DԪ�صķ��ŷֱ���Si��Na��P��N��
��2����������Ԫ���У��ǽ�������ǿ����NԪ�أ���������ǿ����NaԪ�أ���ˣ�����������Ӧˮ����������ǿ����HNO3��������ǿ����NaOH��
��3��N�������ڼ���2���ڣ���һ����������Ԫ���������ﵽ�ȶ��ṹ��ϡ������Ne���縺�ԣ���ϡ�������⣩����Ԫ����F��
��4��EԪ����Fe��ԭ�ӵĺ˵������26��EԪ�������ڱ��ĵ�4���ڵ�VIII�壬��������Χ�����Ų���֪����Ԫ�����ڱ��е�d����
��5��DԪ��ԭ�ӹ��ɵ���ΪN2�������ʽΪ![]() ���÷�������1��������2��������
���÷�������1��������2��������


 ȫ�ŵ�����Ԫ�ƻ�ϵ�д�
ȫ�ŵ�����Ԫ�ƻ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���(Li)��������صĹ���ԭ����ͼ��ʾ����˵������ȷ����
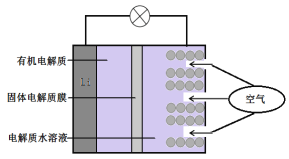
A. �����������������������Ӧ
B. Li+ͨ���л��������ˮ��Һ���ƶ�
C. �����ĵ缫��Ӧ��O2+4e��==2O2��
D. ����ܷ�Ӧ��4Li+O2+2H2O==4LiOH
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪����C2H6��g��![]() C2H4��g��+H2��g�� H1 ��0��
C2H4��g��+H2��g�� H1 ��0��
��C2H6��g��+![]() =2CO2��g��+3H2O��l�� H 2 ��-1559.8 kJ��mol-1
=2CO2��g��+3H2O��l�� H 2 ��-1559.8 kJ��mol-1
��C2H4��g��+3O2��g��=2CO2��g��+2H2O��l�� H 3��-1411.0 kJ��mol-1
����������ȷ����
A.���»��ѹ������ߢ��������ת����
B.���жϼ����յ��������ڳɼ��ų�������
C.��H 2��H 3�ɼ�������е�H
D.�Ʋ�1 mol C2H2��g����ȫȼ�շų�������С��1411.0 kJ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾװ�ã�������ͬ�IJ�������ʢ��NaClϡ��Һ�����з�̪����a��bΪ���ʯī�缫���պ�S1һ��ʱ���a������Һ��죻�Ͽ�S1���պ�S2��������ָ�뷢��ƫת��
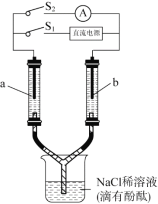
���з�������ȷ����
A.�պ�S1ʱ��a�����ĺ�ɫ��������ɢ
B.�պ�S1ʱ�� a����Һ���b�����ĵ�
C.�Ͽ�S1���պ�S2ʱ��b��������ɫ��dz
D.�Ͽ�S1���պ�S2ʱ��a�Ϸ�����Ӧ��H2 2e- = 2H+
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���о���Դ���������Ȼ����L�ĺϳɶԿ�����ҩ���з�������Ҫ���壬��ϳ�·����Ҫ��Ϊ�����Σ�
I���ϳ��м���F

��֪������TBSClΪ![]()
���� 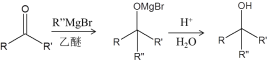
��1��A�������������__________��
��2��B�Ľṹ��ʽ��__________��
��3���Լ�a��__________��
��4��TBSCl��������__________��
II. �ϳ��л���L

��֪�� ![]()
��5��H�к�������������H�Ľṹ��ʽ��__________��
��6��I��J�ķ�Ӧ����ʽ��__________��
��7��K��L��ת���У�������Ӧ�ķ�Ӧ����������__________��__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������������ʵ���Ũ�ȵ����ᡢ����������Һ�ֱ���ڼס������ձ��У���������������������������������Ϊ5:6����ס������ձ��еķ�Ӧ������ֱܷ���
A. �ס����ж��������� B. ���������������м����
C. ��������������������� D. �����������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)���������ķ�Ӧ����������ͬ����������ͬ�����ﲻͬ����Ӧ��ʵ��Ҳ��ͬ���ݴ˻ش��������⣺
�ٳ����£��ڿ������п������ƣ��ƵĶ���������ɫ�䰵��ʧȥ�����������û�ѧ����ʽ�����������������ԭ��__________________��
�����ڿ�����������������Ӧ�Ļ�ѧ����ʽ��__________________��
�۽�4.6����Ͷ������ˮ�У��������������������__________��
(2)����θҺ����θ��(0.2%��0.4%������)����ɱ�����������������ã���θ��������ܹ������٣������������һ����Χ�ڣ���θ�����ʱ��ҽ��ͨ���á�С�մ�Ƭ����θ��ƽ�����������ơ�
����С�մ�Ƭ(NaHCO3)����θ���������ӷ���ʽΪ____________��
���������ͬʱ����θ����ʱ��÷���θ��ƽ[��Ҫ�ɷ���Al(OH)3]����Ӧ�����ӷ���ʽΪ___________________________________��
��ʵ�����Ʊ�Al(OH)3�ij��÷�������Al2(SO4)3��Һ����εμӰ�ˮ����������д����Ӧ�Ļ�ѧ����ʽ��___________________________________��
(3)�����������ʹ�õĽ���֮һ�����������仯�����֪ʶ������������⡣
���й��Ŵ��Ĵ���֮һ��ָ����������Ȼ��ʯ�Ƴɵģ�����Ҫ�ɷ���_______��
��д����ʯ����Ҫ�ɷֺ����ᷴӦ�����ӷ���ʽ______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪ij���������к��� NaCl ���ʣ�Ϊ�ⶨ�����д��������������������ͼ�е�װ�ý���ʵ�顣
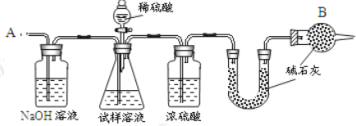
��Ҫʵ�鲽�����£�
�ٰ�ͼ��װ�����������װ�õ������ԣ�
�ڽ� a g ����������ƿ�У�����������ˮ�ܽ⣬�õ�������Һ��
�۳���ʢ�м�ʯ�ҵ� U �ιܵ��������õ� b g��
�ܴӷ�Һ©������ 6 molL-1�����ᣬֱ�����ٲ�������ʱΪֹ��
�ݴӵ��� A ����������һ�����Ŀ�����
���ٴγ���ʢ�м�ʯ�ҵ� U �ܵ��������õ� c g��
���ظ�����ݺ͢IJ�����ֱ�� U �ܵ������������䣬Ϊ d g��
����պͻش����⣺
��1����һ��ϴ��ƿ������������Һ��������____________________
��2��װ���и���� B ��������_______________________________
��3���������Һ©���е����ỻ��Ũ����ͬ�����ᣬ���ԵĽ��_________����ƫ�ߡ�ƫ�ͻ䣩��
��4������ݵ�Ŀ����_______________________________________
��5������ߵ�Ŀ����_________________________________________
��6���������д�������������ļ���ʽΪ________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com