
| X | ||
| Y | ||
| Z |
 ��һ����B��C��ɵĻ�������AC2��Ϊ�ȵ����壬�仯ѧʽΪN2O��
��һ����B��C��ɵĻ�������AC2��Ϊ�ȵ����壬�仯ѧʽΪN2O������ A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E��������DC�ľ���Ϊ���Ӿ��壬D�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��C�γ�-2�������ӣ���Dλ��C����һ���ڣ�B��C���⻯��ķе������ͬ����������Ԫ���⻯��ķе�ߣ������д��������C�γ�-2�������ӣ���CΪ��Ԫ�أ�DΪþԪ�أ��˵����B��C����BΪ��Ԫ�أ�����A��B��C��ͬһ���ڵķǽ���Ԫ�أ�AC2Ϊ�Ǽ��Է��ӣ���AΪ̼Ԫ�أ�E��ԭ������Ϊ24����EΪCrԪ�أ�CrCl3����NH3��H2O�γ�����λ��������������������ʵ���֮��Ϊ2��1������������4��NH3��2��H2O������������λ����磬�������Ϊ[Cr��NH3��4��H2O��2]Cl3��
��� �⣺A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E������DC�ľ���Ϊ���Ӿ��壬D�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��C�γ�-2�������ӣ���Dλ��C����һ���ڣ�B��C���⻯��ķе������ͬ����������Ԫ���⻯��ķе�ߣ�������Ӧ���������C�γ�-2�������ӣ���CΪ��Ԫ�أ�DΪþԪ�أ��˵����B��C����BΪ��Ԫ�أ�����A��B��C��ͬһ���ڵķǽ���Ԫ�أ�AC2Ϊ�Ǽ��Է��ӣ���AΪ̼Ԫ�أ�E��ԭ������Ϊ24����EΪCrԪ�أ�CrCl3����NH3��H2O�γ�����λ��������������������ʵ���֮��Ϊ2��1������������4��NH3��2��H2O������������λ����磬�������Ϊ[Cr��NH3��4��H2O��2]Cl3��
��AΪ̼Ԫ�أ�BΪ��Ԫ�أ�CΪ��Ԫ�أ�DΪþԪ�أ�EΪCrԪ�أ�
��1��AΪ̼Ԫ�ء�BΪ��Ԫ�ء�CΪ��Ԫ�أ�ͬ����������ҵ�һ����������Ԫ��ԭ��2p�ܼ���3�����ӣ����ڰ����ȶ�״̬�����������ͣ���Ԫ�ص�һ�����ܸ������ڵ�Ԫ�صģ����Ե�һ��������С�����˳��ΪC��O��N��
�ʴ�Ϊ��C��O��N��
��2��BΪ��Ԫ�أ����⻯��ΪNH3�������к���3��N-H����Nԭ����1�Թ¶Ե��Ӷԣ��ӻ������Ϊ4��Nԭ�Ӳ�ȡsp3�ӻ����ռ乹��Ϊ�����ͣ�
�ʴ�Ϊ�������ͣ�sp3��
��3��������AC2��CO2��������̼ԭ������ԭ��֮���γ�2�Թ��õ��Ӷԣ�����ʽΪ ��һ����NԪ�ء�OԪ�ػ�������CO2��Ϊ�ȵ����壬�仯ѧʽΪN2O��
��һ����NԪ�ء�OԪ�ػ�������CO2��Ϊ�ȵ����壬�仯ѧʽΪN2O��
�ʴ�Ϊ�� ��N2O��
��N2O��
��4��CrCl3����NH3��H2O�γ�����λ��������������������ʵ���֮��Ϊ2��1������������4��NH3��2��H2O������������λ����磬�������Ϊ��[Cr��NH3��4��H2O��2]Cl3��
�ʴ�Ϊ��[Cr��NH3��4��H2O��2]Cl3��
���� ���⿼��λ�á��ṹ�����ʹ�ϵ���ۺ�Ӧ�ã���Ŀ�Ѷ��еȣ������ۺ��Խϴ��漰�ṹ����Խλ�ù�ϵ��Ԫ�������ɡ�����ʽ���������Ų�����������ӻ����ۡ����ӽṹ��������ԭ��Ӧ��֪ʶ�������ʽṹ���ۺ�����Ŀ���Ƕ�ѧ���ۺ������Ŀ��飬�⻯��ķе������ͬ����������Ԫ���⻯��ķе�����ƶϵ�ͻ�ƿڣ�


 �������ͬ������ϵ�д�
�������ͬ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���ȹ��ˣ����¼�ѹ���� | B�� | �ȳ��³�ѹ���ˣ����¼�ѹ���� | ||
| C�� | �ȳ��¼�ѹ���ˣ����ȹ��� | D�� | ���ζ��ü�ѹ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������ | B�� | ���ޡ����� | C�� | ���ޡ��ߡ����� | D�� | ���١��ڡ��ۡ��ܡ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���Ӱ뾶��Na+��Mg2+��S2-��Cl- | B�� | ���ȶ��ԣ�SiH4��PH3��H2S��HCl | ||
| C�� | ����ǿ����KOH��NaOH��LiOH | D�� | ��ԭ�ԣ�HF��HCl��HBr��HI |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ӻ�ǰ��Ĺ�������䣬���������״�����˸ı� | |
| B�� | sp3��sp2��sp�ӻ�����ļнǷֱ�Ϊ109��28�䡢120�㡢180�� | |
| C�� | �����������Ρ������Ρ�V�η��ӵĽṹ������sp3�ӻ�������� | |
| D�� | �ӻ����ȫ���μ��γɻ�ѧ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
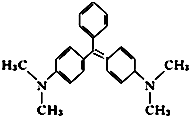 ��ȸʯ���ǻ�����Ʒ�����нϸ߶��ԣ��߲����������°����»��� ��ṹ��ʽ��ͼ��ʾ�����й��ڿ�ȸʯ�̵�˵����ȷ���ǣ�������
��ȸʯ���ǻ�����Ʒ�����нϸ߶��ԣ��߲����������°����»��� ��ṹ��ʽ��ͼ��ʾ�����й��ڿ�ȸʯ�̵�˵����ȷ���ǣ�������| A�� | ��ȸʯ�̵ķ���ʽΪC23H25N2 | |
| B�� | ��ȸʯ�����ڷ����� | |
| C�� | ��ȸʯ�̱����ϵ�һ��ȡ������5�� | |
| D�� | 1mol��ȸʯ����һ��������������6 mol H2�����ӳɷ�Ӧ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com