
ij��ɫ����Һ�п��ܴ�������Ag����Mg2����Cu2����Fe3����Na���еļ��֣�����д���пհף�
(1)�����κ�ʵ��Ϳ��Կ϶�ԭ��Һ�в����ڵ�������________��
(2)ȡ����ԭ��Һ���������ϡ���ᣬ�а�ɫ�������ɣ��ټ��������ϡ���ᣬ��������ʧ��˵��ԭ��Һ�п϶����ڵ�������________���йص����ӷ���ʽΪ______________________________________________________________��
(3)ȡ(2)�е���Һ�����������ϡ��ˮ(NH3��H2O)�����ְ�ɫ������˵��ԭ��Һ�п϶���________���йص����ӷ���ʽΪ____________________________��
(4)ԭ��Һ���ܴ������ڵ������������е�________��
A��Cl�� B��NO3�� C��CO32�� D��OH��
������(1)��ɫ����Һ�в����ܺ���Cu2����Fe3������ɫ���ӡ�(2)��ϡ�����в�����ϡ����İ�ɫ�������ɣ���϶�����Ag�������ӷ���ʽΪAg����Cl��===AgCl����(3)��ϡ��ˮ�а�ɫ�������֣��϶�����Mg2����(4)ԭ��Һ�����ں���Ag�����������в����ܺ���Cl����CO32����OH�������ܺ�NO3����
�𰸡�(1)Cu2����Fe3����(2)Ag����Ag����Cl��== =AgCl��
=AgCl��
(3)Mg2����Mg2����2NH3��H2O===Mg(OH)2����2NH4����(4)B


 �����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���Ż�ʯ��Դ�ļ��٣�����Դ�Ŀ��������������С�
(1)Bunsen�Ȼ�ѧѭ�������������������Ӧ��ɣ�
SO2(g)��I2(g)��2H2O(g)��2HI (g)��H2SO4(l)����������H��a kJ��mol��1
2H2SO4(l)��2H2O(g)��2SO2(g)��O2(g) ��H��b kJ��mol��1
2HI(g)��H2(g)��I2(g) ��H��c kJ��mol��1
��2H2O(g)��2H2(g)��O2(g) ��H�� kJ��mol��1
(2) ��֪��101 kPaʱ��CH4��ȫȼ������1molҺ̬ˮ���ų�������ΪQkJ����CH4��ȫȼ�շ�Ӧ���Ȼ�ѧ����ʽ�ǣ� ��
(3)1molN2(g)��1molO2��g����һ�������·�Ӧ����2molNO(g)������180kJ����������֪����1molN2(g)�е�N��N��1molO2��g���е�O=O�ֱ���Ҫ����946kJ��498kJ����������1molNO�����еĻ�ѧ���γ�ʱ���ͷ� kJ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ӷ���ʽ��д��ȷ���� (����)��
A����SO2ͨ������KMnO4��Һ��2MnO4����5SO2��2H2O===2Mn2����5SO42��
��4H��
B����Na2CO3��Һ�����Ũ�ȵ�����������У�CO32����H��===HCO3��
C���������ƹ�����ˮ��Ӧ��2O22����2H2O===4OH����O2��
D������ˮ��Һ�ʼ��Ե�ԭ��S2����2H2O===H2S��2OH��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������ʵ��װ�ý��е���Ӧʵ�飬�ܴﵽʵ��Ŀ�ĵ���
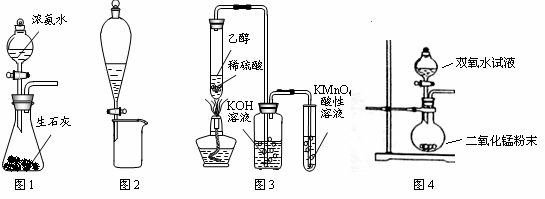
A��ͼ1��ʾװ�ÿ��Ʊ�����
B��ͼ2��ʾװ�ÿɷ���CH3CH2OH��CH3COOC2H5�Ļ��Һ
C��ͼ3��ʾװ�ÿ��Ʊ����ռ���ϩ����֤���ױ�����
D��ͼ4��ʾװ�ÿ��Ʊ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£����и���������ָ����Һ��һ���ܴ���������� (����)��
A��1.0 mol��L��1��KNO3��Һ�У�H����Fe2����Cl����SO42��
B��c(H��)<c(OH��)����Һ�У�Na����K����SO42����ClO��
C����ʹpH��ֽ������Һ�У�K����Ba2����AlO2����Cl��
D��pH��0����Һ�У�Mg2����Na����F����SO42��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ԭ��Ӧ�����ֻ�����Ӧ���͵Ĺ�ϵ��ͼ��ʾ�������л�ѧ��Ӧ������Ӱ���ֵ��� (����)��

A��Cl2��2KBr===Br2��2KCl
B��2NaHCO3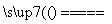 Na2CO3��H2O��CO2��
Na2CO3��H2O��CO2��
C��4Fe(OH)2��O2��2H2O===4Fe(OH)3
D��2Na2O2��2CO2===2Na2CO3��O2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��(Tl)�����軯��(KCN)����ΪA��Σ��Ʒ����֪���з�Ӧ��һ���������ܹ�������(1)Tl3����2Ag===Tl����2Ag����(2)Ag����Fe2��===Ag��Fe3����(3)Fe��
2Fe3��===3Fe2�����������������ԱȽ�˳����ȷ���� (����)��
A��Tl3��>Fe3��>Ag�� B��Fe3��>Ag��>Tl3��
C��Tl��>Ag��>Fe2�� D��Tl3��>Ag��>Fe3��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й��ڻ�ѧ��Ӧ��������˵����ȷ���ǣ� ��
A.��ѧ��Ӧ��һ�������ʱ仯������һ���������仯
B.CaO+H2O =Ca(OH)2�Ƿ��ȷ�Ӧ��˵��CaO ����������Ca(OH)2������
C.Ba(OH)2��8H2O�������Ȼ�茶��巴Ӧ������Ⱦ��ܷ�����˵���÷�Ӧ�Ƿ��ȷ�Ӧ
D.��H2��2H�Ĺ�����Ҫ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ʾ��ѧ֪ʶ������ij�ֹ��ԶԻ�ѧ���ʽ��з��࣬�м����ǵ�Ч�������磬����̼�ظֵĺ�̼���������Ϊ��̼�֡���̼�֡���̼�֣�����������෨�ɽ����ʾΪ��
������һ�������ش�
(1)25 �潫pH����Һ����ԵĹ�ϵ���Եر�ʾ�����������ϣ�
(2)ij��ѧ��ȤС�����о�H2SO4��KCl��NaCl��Na2CO3��Na2SO3��NaOH�������ʵ����ʣ���������о�����������������о�������
���������ǰ����ᡢ��η��࣬Ȼ��ֱ�����ˮ�õ���Һ������ʵ�顣
���������ǰ������Ρ����κ�������������࣬Ȼ��ֱ�����ˮ�õ���Һ������ʵ�顣
�ٸ��ݷ�������з���ʱ����ʵ����KCl��NaCl��Һ��pH����7��H2SO4��Һ��pHС��7��Na2SO3��Na2CO3��NaOH��Һ��pH����7���ɴ��е�ͬѧ�����෨˼���Na2SO3��Na2CO3��NaOH������Ϊ�����Ƿ������Ϊʲô��____________________________________________________________��
���ڷ������У�ijͬѧ�������������е�KCl��NaCl�����������ʻ��ʱ������ͬ�������֣������һ�ּķ�����������������___________ ___________________________________________________________________________________________________________________________ ________��
________��
���ڷ������У��������������������е�________�ɼ������֣��йط�Ӧ�Ļ�ѧ����ʽ�ֱ�Ϊ_________________________________________________
_________________________________________________________________��
�ܸ���ȤС���е�һλͬѧ��Ϊ�������Ը����Ƿ�����Ԫ�ؽ������������ʷ�ΪNa2SO3��Na2CO3��NaCl��NaOH��H2SO4��KCl���ࡣ����H2SO4�������ֺ���Ԫ�ص�����ʱ��Na2SO3��Na2CO3�����õ������֣�������NaCl��NaOHʱȴ�����������������һ��ʵ���������һ���⣺__________ ______________________________________________________________________ ______________________________________________________________
______________________________________________________________
___________________________________________________________________________________________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com