
 £©ÖŠĮłŌŖīŠ½į¹¹Óė±½»·ĄąĖĘ£¬ĖüÓėĻõ»ł±½µÄĻą¶Ō·Ö×ÓÖŹĮæÖ®²īĪŖ3£¬ĘäČŪµćĪŖ354”ę£¬Ļõ»ł±½µÄČŪµćŹĒ5.7”ę£®
£©ÖŠĮłŌŖīŠ½į¹¹Óė±½»·ĄąĖĘ£¬ĖüÓėĻõ»ł±½µÄĻą¶Ō·Ö×ÓÖŹĮæÖ®²īĪŖ3£¬ĘäČŪµćĪŖ354”ę£¬Ļõ»ł±½µÄČŪµćŹĒ5.7”ę£®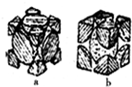
·ÖĪö £Ø1£©18OŌ×Ó»„ĪŖµē×ÓŹżĪŖ8£¬øł¾ŻÄÜĮæ×īµĶŌĄķŹéŠ“ŗĖĶāµē×ÓÅŲ¼Ź½£»·Ē½šŹōŠŌŌ½ĒæµÄŌŖĖŲ£¬ĘäµēøŗŠŌŌ½“ó£»1H218O”¢2H216OµÄ×é³ÉŌŖĖŲĻąĶ¬”¢·Ö×Ó½į¹¹ĻąĶ¬£¬»ÆѧŠŌÖŹ¼øŗõĶźČ«ĻąĶ¬£»
£Ø2£©¢ŁČż¾ŪĒč°·ÖŠ£¬»·ÉĻÓė»·ĶāµÄµŖŌ×Ó·Ö±šŠĪ³É2øö¦Ņ¼ü”¢3øö¦Ņ£¬¾łŗ¬ÓŠ1¶Ō¹Ā¶Ōµē×Ó£¬ŌӻƹģµĄŹżÄæ·Ö±šĪŖ3”¢4£»
¢ŚČż¾ŪĒč°··Ö×ÓÖ®¼äŠĪ³ÉĒā¼ü£¬Ōö“óĪļÖŹµÄČŪµć£»
£Ø3£©CuĪ»ÓŚµŚĖÄÖÜĘŚIB×壬ŹōÓŚdsĒųŌŖĖŲ£»ĻąĶ¬Ģõ¼žĻĀĘųĢåµÄĆܶČÖ®±ČµČÓŚĘäĻą¶Ō·Ö×ÓÖŹĮæÖ®±Č£¬¹ŹXµÄĻą¶Ō·Ö×ÓÖŹĮæĪŖ135”Į2=270£¬ŌņXµÄ·Ö×ÓŹ½ĪŖCu2Cl2£¬ŹōÓŚ·Ö×Ó¾§Ģ壬XČÜÓŚŃĪĖįÖŠæÉŠĪ³ÉH2[CuCl2]µÄĖį£¬øĆĖįÖŠ³żĘÕĶصĹ²¼Ū¼üĶā£¬»¹“ęŌŚÅäĪ»¼ü£»
£Ø4£©ÓÉĶ¼æÉÖŖ£¬aĪŖĆęŠÄĮ¢·½×īĆܶѻż£¬bĪŖĢåŠÄĮ¢·½×īĆܶѻż£»ĄūÓŚ¾łĢƷؼĘĖćb¾§°ūÖŠFeŌ×ÓŹżÄ棬±ķŹ¾³ö¾§°ūÖŹĮ棬FeŌ×ӵİė¾¶ĪŖr pm£¬¶ų“¦ÓŚĢå¶Ō½ĒĻßÉĻŌ×ÓĻąĒŠ£¬¹Ź¾§°ūĄā³¤ĪŖ$\frac{4r}{\sqrt{3}}$ pm£¬ŌŁøł¾Ż¦Ń=$\frac{m}{V}$¼ĘĖć£®
½ā“š ½ā£ŗ£Ø1£©18OŌ×Ó»„ĪŖµē×ÓŹżĪŖ8£¬ŗĖĶāµē×ÓÅŲ¼Ź½ĪŖ1s22s22p4£»·Ē½šŹōŠŌŌ½ĒæµÄŌŖĖŲ£¬ĘäµēøŗŠŌŌ½“󣬹ŹµēøŗŠŌO£¾S£¾C£¾H£»1H218O”¢2H216OµÄ×é³ÉŌŖĖŲĻąĶ¬”¢·Ö×Ó½į¹¹ĻąĶ¬£¬»ÆѧŠŌÖŹ¼øŗõĶźČ«ĻąĶ¬£¬
¹Ź“š°øĪŖ£ŗ1s22s22p4£»O£¾S£¾C£¾H£»¼øŗõĶźČ«ĻąĶ¬£»
£Ø2£©¢ŁČż¾ŪĒč°·ÖŠ£¬»·ÉĻÓė»·ĶāµÄµŖŌ×Ó·Ö±šŠĪ³É2øö¦Ņ¼ü”¢3øö¦Ņ£¬¾łŗ¬ÓŠ1¶Ō¹Ā¶Ōµē×Ó£¬ŌӻƹģµĄŹżÄæ·Ö±šĪŖ3”¢4£¬ŌӻƹģµĄĄąŠĶ·ÖeĪŖsp2”¢sp3Ōӻƣ¬
¹Ź“š°øĪŖ£ŗsp2”¢sp3£»
¢ŚČż¾ŪĒč°·ÖŠ“ęŌŚN-H¼ü£¬·Ö×Ó¼äÄÜŠ“³öĒā¼ü£¬µ¼ÖĀČŪµćÉżøߣ¬Ļõ»ł±½·Ö×ÓÖ®¼ä²»ÄÜŠĪ³ÉĒā¼ü£¬¹ŹČŪµć½ĻµĶ£¬
¹Ź“š°øĪŖ£ŗČż¾ŪĒč°·ÖŠ“ęŌŚN-H¼ü£¬·Ö×Ó¼äÄÜŠ“³öĒā¼ü£¬µ¼ÖĀČŪµćÉżøߣ¬Ļõ»ł±½·Ö×ÓÖ®¼ä²»ÄÜŠĪ³ÉĒā¼ü£¬¹ŹČŪµć½ĻµĶ£»
£Ø3£©CuĪ»ÓŚµŚĖÄÖÜĘŚIB×壬ŹōÓŚdsĒųŌŖĖŲ£»ĻąĶ¬Ģõ¼žĻĀĘųĢåµÄĆܶČÖ®±ČµČÓŚĘäĻą¶Ō·Ö×ÓÖŹĮæÖ®±Č£¬¹ŹXµÄĻą¶Ō·Ö×ÓÖŹĮæĪŖ135”Į2=270£¬ŌņXµÄ·Ö×ÓŹ½ĪŖCu2Cl2£¬ŹōÓŚ·Ö×Ó¾§Ģ壬XČÜÓŚŃĪĖįÖŠæÉŠĪ³ÉH2[CuCl2]µÄĖį£¬øĆĖįÖŠ³żĘÕĶصĹ²¼Ū¼üĶā£¬»¹“ęŌŚÅäĪ»¼ü£¬
¹Ź“š°øĪŖ£ŗds£»Cu2Cl2£»·Ö×Ó¾§Ģ壻ÅäĪ»£»
£Ø4£©ÓÉĶ¼æÉÖŖ£¬aĪŖĆęŠÄĮ¢·½×īĆܶѻż£¬bĪŖĢåŠÄĮ¢·½×īĆܶѻż£¬b¾§°ūÖŠFeŌ×ÓŹżÄæĪŖ1+8”Į$\frac{1}{8}$=2£¬Ōņ¾§°ūÖŹĮæĪŖ2”Į$\frac{56}{{N}_{A}}$g£¬FeŌ×ӵİė¾¶ĪŖr pm£¬¶ų“¦ÓŚĢå¶Ō½ĒĻßÉĻŌ×ÓĻąĒŠ£¬¹Ź¾§°ūĄā³¤ĪŖ$\frac{4r}{\sqrt{3}}$ pm£¬¹Ź¾§ĢåµÄĆܶČĪŖ2”Į$\frac{56}{{N}_{A}}$g”Ā£Ø$\frac{4r}{\sqrt{3}}$”Į10-10 cm£©3=$\frac{21\sqrt{3}”Į1{0}^{30}}{4{r}^{3}{N}_{A}}$g/cm3£¬
¹Ź“š°øĪŖ£ŗa£»$\frac{21\sqrt{3}”Į1{0}^{30}}{4{r}^{3}{N}_{A}}$£®
µćĘĄ ±¾ĢāŹĒ¶ŌĪļÖŹ½į¹¹ÓėŠŌÖŹµÄ漲飬Éę¼°ŗĖĶāµē×ÓÅŲ¼”¢µēøŗŠŌ”¢ŌӻƷ½Ź½”¢Ēā¼ü”¢ÅäŗĻĪļ”¢¾§ĢåĄąŠĶÓėŠŌÖŹ”¢¾§°ū¼ĘĖćµČ£¬ŹĒ¶ŌĪļÖŹ½į¹¹Ö÷øÉÖŖŹ¶µÄ漲飬ŠčŅŖѧɜ¾ß±øŌśŹµµÄ»ł“”£¬£Ø4£©ÖŠ¹Ų¼üŹĒĆ÷Č·¾§°ūĄā³¤ÓėŌ×Ó°ė¾¶¹ŲĻµ£®


 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¼×Ķ锢ŅŅČ²”¢ŅŅĻ©”¢±ūĻ© | B£® | ±½”¢¼×±½”¢¼ŗĶé | ||
| C£® | CH3CHO”¢CH3COOH”¢CH3CH2OH | D£® | C6H5OH”¢C6H5C2H5”¢C6H6 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¶ąŃ”Ģā
| A£® | CH4 | B£® | C2H6 | C£® | C3H8 | D£® | C3H6 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŹŅĪĀĻĀ£¬²āµĆ0.1 mol/L µÄ°±Ė®µÄpHĪŖ11£ŗNH3•H2O?NH4++OH- | |
| B£® | ĖįŠŌ½éÖŹÖŠKMnO4Ńõ»ÆH2O2£ŗ2MnO4-+5H2O2+6H+ØT2Mn2++5O2”ü+8H2O | |
| C£® | Ļņ NaAlO2ČÜŅŗÖŠĶØČė¹żĮæCO2 ÖĘ Al£ØOH£©3£ŗCO2+AlO2-+2H20=HC03-+Al£ØOH£©3”ż | |
| D£® | Ē¦ĖįŠīµē³ŲŌŚ³äµēŹ±µÄÕż¼«·“Ó¦£ŗPbO2+4H++SO42-+2e-ØTPbSO4+2H2O |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĆŗÖŠ“ęŌŚ±½”¢¶ž¼×±½£¬¹¤ŅµÉĻæÉÓÉĆŗÕōĮó»ńµĆ | |
| B£® | Ö±ĮóĘūÓĶŗĶĮŃ»ÆĘūÓĶÖŠ·Ö±š¼ÓČėäåµÄĖÄĀČ»ÆĢ¼ČÜŅŗ¾łÄÜ·¢Éś»Æѧ·“Ó¦ | |
| C£® | ŹÆÓĶµÄĮŃ»Æ”¢ĮŃ½āŗĶĆŗµÄøÉĮó”¢Ęų»Æ¶¼ŹōÓŚ»Æѧ±ä»Æ | |
| D£® | ŹÆÓĶĮŃ½āŹĒÉś²śŅŅĻ©µÄÖ÷ŅŖ·½·Ø£¬ŅŅĻ©ŗĶ¾ŪŅŅĻ©¾łÄÜŹ¹äåĖ®·¢Éś·“Ó¦¶ųĶŹÉ« |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | NaCl HCl H2O NaOH | B£® | Cl2Na2S HCl CO2 | ||
| C£® | HBr CCl4H2O CO2 | D£® | Na2O2H2O2H2O O2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com