
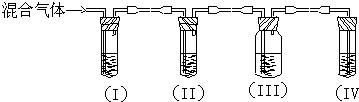
·ÖĪö £Ø1£©øł¾ŻŹµŃéµÄÄæµÄŹĒ¼ģŃéŅŅĻ©ŗĶ¶žŃõ»ÆĮņ£¬ĖłŅŌŠčŅŖĻČ¼ģŃéŅŅĻ©ÖŠŗ¬ÓŠ¶žŃõ»ÆĮņ£¬Č»ŗó³żČ„ŌÓÖŹ¶žŃõ»ÆĮņ£¬ŌŁ¼ģŃéŅŅĻ©£»
£Ø2£©øł¾Ż¶žŃõ»ÆĮņÄÜĘÆ°×Ę·ŗģ½ųŠŠ¼ģŃ飻øł¾ŻĒāŃõ»ÆÄĘČÜŅŗÄÜÓė¶žŃõ»ÆĮņ·“Ó¦µÄŠŌÖŹ·ÖĪö£»¼ģŃéŅŅĻ©Ē°£¬±ŲŠėĻČČ·ČĻŅŅĻ©ÖŠµÄSO2ŅŃ³żøɾ»£»¢óÖŠĪŽ¶žŃõ»ÆĮņ£¬¢ōÖŠÓėäåĖ®·“Ó¦µÄŹĒŅŅĻ©£®
½ā“š ½ā£ŗ£Ø1£©¼ģŃ鶞Ńõ»ÆĮņÓĆĘ·ŗģČÜŅŗ£¬¼ģŃéŅŅĻ©ÓĆäåĖ®£¬ŅŅĻ©ŗĶ¶žŃõ»ÆĮņ¶¼ÄÜŹ¹äåĖ®ĶŹÉ«£¬ĖłŅŌĻČ¼ģŃ鶞Ńõ»ÆĮņ£¬Č»ŗó¼ģŃéŅŅĻ©£¬Ķ¬ŌŚ¼ģŃéŅŅĻ©Ö®Ē°ÓĆNaOHČÜŅŗ³ż¾”SO2£¬ŌŁĶعżĘ·ŗģČÜŅŗ²»ĶŹÉ«Č·ČĻSO2ŅŃ³żøɾ»£¬×īŗóÓĆäåĖ®¼ģŃéŅŅĻ©£¬
Ņņ×°ÖĆIÓĆĄ“¼ģŃéSO2£¬ŹŌ¹ÜÖŠĘ·ŗģČÜŅŗĶŹÉ«£¬ĖµĆ÷ŗ¬ÓŠSO2£¬×°ÖĆIIŹŌ¹Ü×°ÓŠNaOHČÜŅŗ³żČ„SO2£¬×°ÖĆIIIŹŌ¹ÜĶعżĘ·ŗģČÜŅŗ²»ĶŹÉ«Č·ČĻSO2ŅŃ³żøɾ»£¬×°ÖĆIVĶعżäåĖ®ĶŹÉ«¼ģŃéŅŅĻ©£¬
¹Ź“š°øĪŖ£ŗA£»B£»A£»D£»
£Ø2£©×°ÖĆIÓĆĄ“¼ģŃéŹĒ·ń“ęŌŚSO2£¬ŹŌ¹ÜÖŠĘ·ŗģČÜŅŗĶŹÉ«£¬ĖµĆ÷ŗ¬ÓŠSO2£»
ĒāŃõ»ÆÄĘČÜŅŗÄܹ»Óė¶žŃõ»ÆĮņ·“Ó¦£¬ĖłŅŌ×°ÖĆ¢ņŹŌ¹Ü×°ÓŠNaOHČÜŅŗ³żČ„SO2£»
Ķعż×°ÖĆ¢óŹŌ¹ÜÖŠĶعżĘ·ŗģČÜŅŗ²»ĶŹÉ«£¬Č·ČĻSO2ŅŃ³żøɾ»£»
×°ÖĆ¢ōĶعżäåĖ®ĶŹÉ«¼ģŃéŅŅĻ©£¬
¹Ź“š°øĪŖ£ŗ¢ńÖŠĘ·ŗģČÜŅŗĶŹÉ«£»³żČ„¶žŃõ»ÆĮņŅŌĆāøÉČÅŅŅĻ©µÄ¼ģŃ飻¼ģŃ鶞Ńõ»ÆĮņŹĒ·ń±»ĶźČ«³żČ„£»¢óÖŠµÄĘ·ŗģ²»ĶŹÉ«£¬¢ōÖŠµÄøßĆĢĖį¼ŲČÜŅŗĶŹÉ«£®
µćĘĄ ±¾Ģāæ¼²éĮĖŅŅĻ©µÄŹµŃéŹŅÖĘ·ØŅŌ¼°²śĪļµÄ¼ģŃ飬ĢāÄæÄѶČÖŠµČ£¬×¢Ņāµ±ÓŠ¶ąÖÖ²śĪļŠč¼ģŃ鏱£¬Ó¦æ¼ĀĒĻČŗóĖ³Šņ£»äåĖ®¼ČÄÜŃõ»Æ¶žŃõ»ÆĮņ£¬ÓÖÄÜÓėŅŅĻ©·¢Éś¼Ó³É·“Ó¦£¬¼ģŃéŅŅĻ©Ē°±ŲŠė³żČ„¶žŃõ»ÆĮņ£®


 ĆūŠ£æĪĢĆĻµĮŠ“š°ø
ĆūŠ£æĪĢĆĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | CH4 | B£® | C2H2 | C£® | C2H4 | D£® | C2H6 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | WX2·Ö×ÓÖŠĖłÓŠŌ×Ó×īĶā²ć¶¼ĪŖ8 µē×Ó½į¹¹ | |
| B£® | WX2”¢ZX2µÄ»Æѧ¼üĄąŠĶĻąĶ¬ | |
| C£® | WX2ŹĒŅŌ¼«ŠŌ¼ü½įŗĻ³ÉµÄ·Ö×Ó | |
| D£® | Ō×Ó°ė¾¶“óŠ”Ė³ŠņĪŖX£¼W£¼Y£¼Z |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
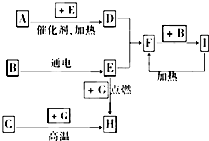 ĻĀĮŠŹĒ֊ѧ»Æѧ֊³£¼ūĪļÖŹ¼äµÄ·“Ó¦×Ŗ»Æ¹ŲĻµĶ¼£¬ĘäÖŠ²æ·Ö²śĪļŅŃĀŌČ„£¬³£ĪĀĻĀ£¬GĪŖ¹ĢĢåµ„ÖŹ£¬B”¢IĪŖŅŗĢ壬ĘäÓą¶¼ĪŖĘųĢ壮AĪŖ»ÆŗĻĪļ£¬IµÄÅØČÜŅŗÓėGŌŚ¼ÓČČĢõ¼žĻĀÉś³ÉF”¢BŗĶC£® HæÉÓĆ×÷¹¤ŅµÉĻŅ±Į¶½šŹōµÄ»¹Ō¼Į£®Ēė°“ŅŖĒóĢīæÕ£ŗ
ĻĀĮŠŹĒ֊ѧ»Æѧ֊³£¼ūĪļÖŹ¼äµÄ·“Ó¦×Ŗ»Æ¹ŲĻµĶ¼£¬ĘäÖŠ²æ·Ö²śĪļŅŃĀŌČ„£¬³£ĪĀĻĀ£¬GĪŖ¹ĢĢåµ„ÖŹ£¬B”¢IĪŖŅŗĢ壬ĘäÓą¶¼ĪŖĘųĢ壮AĪŖ»ÆŗĻĪļ£¬IµÄÅØČÜŅŗÓėGŌŚ¼ÓČČĢõ¼žĻĀÉś³ÉF”¢BŗĶC£® HæÉÓĆ×÷¹¤ŅµÉĻŅ±Į¶½šŹōµÄ»¹Ō¼Į£®Ēė°“ŅŖĒóĢīæÕ£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 £®
£® £¬øĆ¾§ĢåÖŠŗ¬Ī¢Į£¼äµÄ×÷ÓĆÓŠ£ŗĄė×Ó¼üŗĶ·Ē¼«ŠŌ¼ü£®
£¬øĆ¾§ĢåÖŠŗ¬Ī¢Į£¼äµÄ×÷ÓĆÓŠ£ŗĄė×Ó¼üŗĶ·Ē¼«ŠŌ¼ü£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
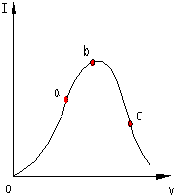 ŌŚŅ»¶ØĪĀ¶ČĻĀ£¬±ł“×Ėį¼ÓĖ®Ļ”ŹĶ¹ż³ĢÖŠ£¬ČÜŅŗµÄµ¼µēÄÜĮ¦IĖę¼ÓČėĖ®µÄĢå»żV±ä»ÆµÄĒśĻßČēĶ¼ĖłŹ¾£®Ēė»Ų“š£ŗ
ŌŚŅ»¶ØĪĀ¶ČĻĀ£¬±ł“×Ėį¼ÓĖ®Ļ”ŹĶ¹ż³ĢÖŠ£¬ČÜŅŗµÄµ¼µēÄÜĮ¦IĖę¼ÓČėĖ®µÄĢå»żV±ä»ÆµÄĒśĻßČēĶ¼ĖłŹ¾£®Ēė»Ų“š£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā

²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com