
��֪X��Y��Z��W��R��ԭ������������������ֶ���������Ԫ�أ�����Y��Rԭ��������������ȣ�XԪ���������WԪ��������۾���ֵ��ȣ���ҵ�ϳ��õ������������ķ���ұ��W���ʣ�Z��W��R����������Ӧ��ˮ����������Ӧ�������κ�ˮ������˵����ȷ����
A�������Ӱ뾶��Y��Z��W
B�����⻯������ȶ��ԣ�X��Y��R
C��W������������Ӧ��ˮ������Ա�Z��ǿ
D��R������������Ӧ��ˮ���ﻯѧʽһ����HRO4

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ��ɽ��ʡ�����и����ڶ���ģ�⻯ѧ�Ծ��������棩 ���ͣ������
�����������ʵ���Ҫ���������ʽṹ����ش��������⡣
��1����ͼ��ʯī�Ľṹ���侧���д��ڵ��������� ������ţ�
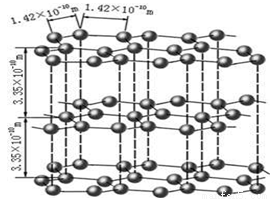
A���Ҽ�
B���м�
C�����
D�����
E�����Ӽ�������
F��������
G�����Ӽ�
��2��������ھ����˵������ȷ����___________
A�������۵��ɵ͵��ߣ�CF4��CCl4��CBr4��CI4
B��Ӳ���ɴ�С�����ʯ��̼���裾�����
C���۵��ɸߵ��ͣ�Na��Mg��Al
D���������ɴ�С��NaF��NaCl��NaBr��NaI
��3��CaF2�ṹ��ͼ����ʾ��Cu�γɾ���Ľṹ�����ʾ����ΪH3BO3����ṹͼ����״�ṹ�����ڵ�H3BO3����ͨ�������ϣ�
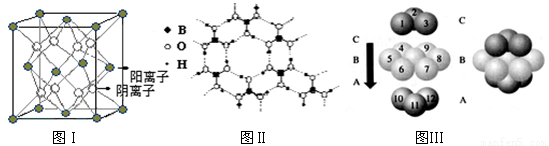
��ͼI��ʾ�ľ�������Ca2+��������ҵȾ����Ca2+������Ϊ ��ͼIII��δ��ŵ�Cuԭ���γɾ������Χ����ڵ�Cuԭ����Ϊ ��
��H3BO3������Bԭ���ӻ���ʽ______;
�����־������۵�ߵ͵�˳��Ϊ ���ѧʽ����H3BO3���������ۻ�ʱ���˷�����֮��������Ϊ_______________��
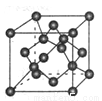
��4��̼��ij�ֵ��ʵľ�����ͼ��ʾ��һ����������_____��̼ԭ�ӣ����þ�����ܶ�Ϊ�� g/cm3�������ӵ�������ֵΪNA�����������������̼ԭ��֮��ľ���Ϊ_ ____cm���ô���ʽ��ʾ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�꽭��ʡ̩���и����ڶ���ģ�⿼�Ի�ѧ�Ծ��������棩 ���ͣ��ƶ���
(15��)������F�Ǻϳɿ�����ҩ��³˾���Ƶ���Ҫ�м��壬��ϳɹ������£�
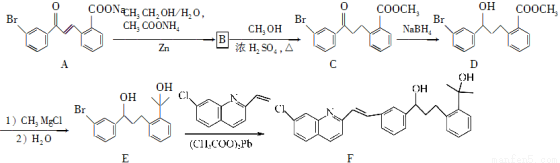
��ش��������⣺
��1��������C�к���������Ϊ________��________(������)��
��2��������B�ķ���ʽΪC16H13O3Br����B�Ľṹ��ʽΪ____________��
��3����C��D��E��F�ķ�Ӧ��������Ϊ________��________��
��4��д��������������C��һ��ͬ���칹��Ľṹ��ʽ��______________��
�����ڷ����廯����ҷ����к���2��������
���ܹ�����������Ӧ��
��������5�ֲ�ͬ��������ԭ�ӡ�
��5����֪��RCl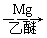 RMgCl��д����CH3CH2OH��
RMgCl��д����CH3CH2OH��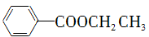 Ϊԭ���Ʊ�
Ϊԭ���Ʊ�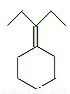 �ĺϳ�·������ͼ(�����ܼ������Լ�����)���ϳ�·������ͼʾ�����£�
�ĺϳ�·������ͼ(�����ܼ������Լ�����)���ϳ�·������ͼʾ�����£�
H2C��CH2 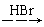 CH3CH2Br
CH3CH2Br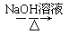 CH3CH2OH
CH3CH2OH
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�꽭��ʡ��ͨ�����ݡ����Ƹ۸����ڶ��ε��в��Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
B��[ʵ�黯ѧ]���IJ��к��зḻ��ά����C��ά����C��������Һ���ܱ���������ijʵ��С��ͨ������ʵ��Ծ��IJ���ά����C�ĺ������вⶨ��
��һ��������ά����C����Һ����5Ƭ100 mg��ά����CҩƬ���顢�ܽ⣬���250 mL����Һ��
�ڶ�������ȡ���IJ�֭��ȡ50 g���ʵľ��IJˣ����ã���ˮ��ֽ��裬����ͼ��ʾװ�ó����Ƶþ��IJ�֭50 mL��
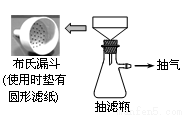
������������Һ��ά����C�����IJⶨ����ȡ20.00 mLά����C����Һ����ƿ�У�����1 mL 0.1 mol��L��1�����ữ������2�ε�����Һ��ָʾ������0.010 mol��L��1��ˮ�ζ����յ㣬��¼���ĵ�ˮ��������ظ������������Σ����ĵ�ˮ��ƽ�����ΪV1��
���IJ������IJ�֭��ά����C�����IJⶨ����ȡ20 mL���IJ�֭����ƿ�У�����1 mL 0.1 mol��L��1�����ữ������2�ε�����Һ��ָʾ������0.010 mol��L��1��ˮ�ζ����յ㣬��¼���ĵ�ˮ��������ظ������������Σ����ĵ�ˮ��ƽ�����ΪV2��
��1���������ȣ����˵��ŵ��� ���������õ���ֽӦ�� ������ڡ���С�ڡ�������©���ھ�����ȫ��С��ס��
��2����ȡ20.00 mL����Һѡ�õ������� ���ζ��յ���Һ����ɫ�� ɫ��
��3�������ζ����̵ζ��ٶ�Ҫ�죬����ʹ��õ�ά����C�ĺ���ƫ�ͣ�����ܵ�ԭ���� ��
��4��1kg���IJ�������ά����C�൱�� Ƭ����ά����ҩƬ�����ú�V1��V2�Ĵ���ʽ��ʾ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�꽭��ʡ��ͨ�����ݡ����Ƹ۸����ڶ��ε��в��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��2 L�ĺ����ܱ������г���A(g)��B(g)��������Ӧ��
A(g)��B(g)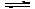 2C(g)��D(s) ��H��a kJ��mol��1
2C(g)��D(s) ��H��a kJ��mol��1
ʵ�����ݺͽ���ֱ����±�����ͼ��ʾ������˵����ȷ����
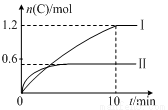
ʵ����� | �¶� | ��ʼ���ʵ��� | �����仯 | |
A | B | |||
�� | 600�� | 1 mol | 3 mol | 96 kJ |
�� | 800�� | 1.5 mol | 0.5 mol | ���� |
A��ʵ����У�10 min��ƽ������v(B)��0.06 mol��L��1��min��1
B����������ʽ��a����160
C��600 ��ʱ���÷�Ӧ��ƽ�ⳣ����0.45
D����ʵ����ƽ����ϵ���ٳ���0.5 mol A��1.5 mol B��A��ת��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�꽭��ʡ��ͨ�����ݡ����Ƹ۸����ڶ��ε��в��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
25��ʱ�����и���������ָ����Һ��һ���ܴ����������
A��ʹ��̪���ɫ����Һ��NH4����Ca2����NO3����SO42��
B��c(OH��)��1��10��13 mol��L��1����Һ��Na����K����ClO����SO42��
C��0.1 mol��L��1 FeCl2��Һ��K����Na����SO42����NO3��
D��0.1 mol��L��1 KMnO4��Һ��Na����Mg2����NO3����SO32��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�꼪��ʡ�����и���������������ۻ�ѧ�Ծ��������棩 ���ͣ������
ѡ��[��ѧ��ѡ��2����ѧ�뼼��]��15�֣���ҵ�ϳɰ������Ƕ�����ľ���֮һ�������Ǻϳɰ��ļ�Ҫ����ʾ��ͼ��
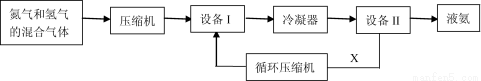
��1���豸I�������� ����X·��ѭ���������� ������ĸ����
A.N2��H2 B.���� C.NH3 D.N2��H2��NH3
��2���ϳɰ���H2�����ɽ�̿��ˮ������Ӧ��á���д���÷�Ӧ�Ļ�ѧ����ʽ��_______��
��3��ԭ��������������H2S��CO��CO2�����ʣ������ȥ��Щ���ʵ�Ŀ���� ������K2CO3��Һ��ȥCO2���䷴Ӧ�����ӷ���ʽΪ ��
��4���������˽���ų�ֱ�Ӽ��ڵ�����������Ӧ�������ڣ��ڽϵ͵��¶Ⱥ�ѹǿ�����ºϳɰ�������˽ϸߵIJ��ʡ���ų��Ժϳɰ���Ӧ���ʵ�Ӱ����_______���÷������ŵ���_________________��
��5���ϳɰ���ҵ�������д���������ˮ��Ϊ�˷�ֹ�Ի��������Ⱦ���������õ�ⷨ��NH3��H2Oת��Ϊ�Ի�������Ⱦ�ļ��Դ��������ʱ��ʯī���缫����������Ϊ����ʣ��������ϴ���1mol NH3��H2O����·��ת�Ƶ��� mol�������ĵ缫��ӦʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�����ʡ�����и������µ��п������ۻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
����ʵ�顢������ؽ��۾���ȷ����
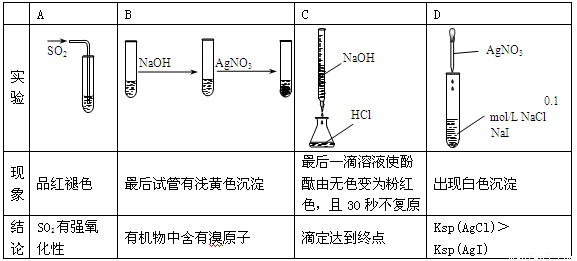
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ���������У����4��������ѧ�Ծ��������棩 ���ͣ�ѡ����
��ѧ���������������أ�����˵����ȷ����
A���������Ϊ95%�ľƾ�ͨ����Ϊҽ�þƾ�
B��ʯ�͵ķ���ú����������ˮ����þ��������ѧ�仯
C����ɫ��ѧ�ĺ�����Ӧ�û�ѧԭ���Ի�����Ⱦ��������
D�����øߴ��ȹ������̫���ܵ�ذ�ɽ�����ֱ��ת��Ϊ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com