���������Բ�������ϳɷ�����ȥ������ԭ�ϣ�
���ݰ����ӵ����ɵ���������ܶ�֮�ȵ���Ħ������֮�ȣ�E������������������ܶ�Ϊ30��Ħ��������60���������ɶ�����̼��ˮ������ȷ�����ʽ�����Ħ��������ȷ���л���ΪC
2H
4O
2��Ϊ���ᣬ�ɿ�ͼ��֪E��C��������F����ԭ���غ�֪C�ķ���ʽ��C
8H
10O����ΪAΪһȡ����������B�к���һ����������B�Ľṹ��ʽΪC
6H
5CHClCH
3��D������Br
2�����ӳɷ�Ӧ������D�DZ���ϩ���ṹ��ʽΪC
6H
5CH=CH
2��C��C
6H
5CHOHCH
3�������������Ľṹ��ʽ��

����������Ϣ���л���Ľṹ�����ʿɽ����⣮
����⣺������Բ�������ϳɷ�������

������

�������л��������ԭ������ԭ�Ϸֱ���2��3-����-l��3-����ϩ�ͱ�Ȳ������2-��-l��3-����ϩ��2-��Ȳ���ʴ�Ϊ��AD��
��1�����ݰ����ӵ����ɵ���������ܶ�֮�ȵ���Ħ������֮�ȣ����EĦ��������60.6.0gE�����ʵ�������0.1mol����ȫȼ�պ�����C0
2�� H
20�����ʵ����ֱ�Ϊ
=0.2mol��
=0.2mol������̼����������ֱ�Ϊ0.2��12=2.4g��0.4��1=0.4g�����E����Ԫ�ص�����Ϊ6.0-2.4-0.4=3.2g��������Ԫ�ص����ʵ���Ϊ
=0.2mol�����̼���⡢������ԭ�Ӹ���֮��Ϊ1��2��1�������ʽΪCH
2O����ΪEĦ��������60�����Է���ʽ��C
2H
4O
2��
�ʴ�Ϊ��C
2H
4O
2��
��2���ɿ�ͼ��֪E��C��������F����ԭ���غ�֪C�ķ���ʽ��C
8H
10O����ΪAΪһȡ����������B�к���һ����������B�Ľṹ��ʽΪC
6H
5CHClCH
3��B����C�Ļ�ѧ����ʽ�ǣ�C
6H
5CHClCH
3+H
2O
C
6H
5CHOHCH
3+HCl��
�ʴ�Ϊ��C
6H
5CHClCH
3+H
2O
C
6H
5CHOHCH
3+HCl��
��3����ΪD������Br
2�����ӳɷ�Ӧ������D�DZ���ϩ���ṹ��ʽΪC
6H
5CH=CH
2��±����������ȥ��Ӧ��������NaOH����Һ�����ȣ���������ȥ��Ӧ��������Ũ���Ტ���ȣ�
�ʴ�Ϊ��NaOH����Һ�����ȣ�Ũ���Ტ���ȣ�
��4��A���ڱ���ͬϵ�B����±������������A����B�ķ�Ӧ������ȡ����Ӧ��D�к���̼̼˫���������D����G�ķ�Ӧ�����Ǽӳɷ�Ӧ���ʴ�Ϊ��ȡ����Ӧ���ӳɷ�Ӧ��
��5����ΪC��E���Է���������Ӧ������E�����ᣬ����ΪC��C
6H
5CHOHCH
3�������������Ľṹ��ʽ��

��
�ʴ�Ϊ��

��
��6��������һ�����IJ���ֻ��һ�֣�˵��Ӧ���ǶԳ��Խṹ������G�ķ���ʽC
8H
8Br
2��֪���������Ĺ�������7�֣�

���к˴Ź�������������壬�ҷ������Ϊl��1����

��
�ʴ�Ϊ��7��

��

 �����Ҫ�ϳ�
�����Ҫ�ϳ�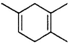 ���õ�ԭʼԭ�Ͽ�����
���õ�ԭʼԭ�Ͽ�����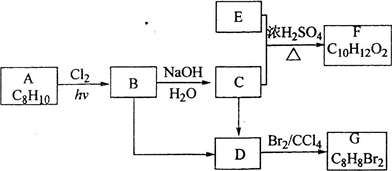




 ����������Ϣ���л���Ľṹ�����ʿɽ����⣮
����������Ϣ���л���Ľṹ�����ʿɽ����⣮ ������
������ �������л��������ԭ������ԭ�Ϸֱ���2��3-����-l��3-����ϩ�ͱ�Ȳ������2-��-l��3-����ϩ��2-��Ȳ���ʴ�Ϊ��AD��
�������л��������ԭ������ԭ�Ϸֱ���2��3-����-l��3-����ϩ�ͱ�Ȳ������2-��-l��3-����ϩ��2-��Ȳ���ʴ�Ϊ��AD�� ��
�� ��
��
 ��
�� ��
��