
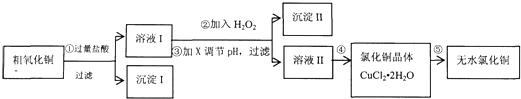
��16�֣�ijͬѧ���ô�����ͭ������������������������������ʣ���ȡ��ˮ�Ȼ�ͭ��������ͼ��ʾ��
��1����������漰�����ӷ���ʽ�У� ��
��2��������м���H2O2��Ŀ���ǣ� ��Ӧ�����ӷ���ʽΪ ��
��3����֪��
| �� | �������↑ʼ����ʱ��pH | �������������ȫʱ��pH |
| Fe3�� | 1.9 | 3.2 |
| Cu2�� | 4.7 | 6.7 |
| Fe2�� | 7 | 9 |
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �������↑ʼ����ʱ��pH | �������������ȫʱ��pH | |
| Fe3+ | 1.9 | 3.2 |
| Cu2+ | 4.7 | 6.7 |
| Fe2+ | 7 | 9 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��㶫ʡ�����и�����һ�ε��п��������ۺϻ�ѧ�Ծ����������� ���ͣ������
��16�֣�ijͬѧ���ô�����ͭ��������FeO��������������ʣ���ȡ��ˮ�Ȼ�ͭ��������ͼ��
(1)�����������ͭ�����ᷴӦ���������ʽ��___________________��
(2)������м���H2O2��Ŀ��____________________________________��
����II�Ļ�ѧʽΪ_______________________��
(3)��֪��
| | �������↑ʼ����ʱ��pH | �������������ȫʱ��pH |
| Fe3+ | 1.9 | 3.2 |
| Cu2+ | 4.7 | 6.7 |
| Fe2+ | 7 | 9 |

 ��ԭ����__________________________________________________________��
��ԭ����__________________________________________________________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��㶫ʡ������һ��8���¿������ۺϻ�ѧ�Ծ��������棩 ���ͣ������
��16�֣�ijͬѧ���ô�����ͭ������������������������������ʣ���ȡ��ˮ�Ȼ�ͭ��������ͼ��ʾ��

��1����������漰�����ӷ���ʽ�У� ��
��2��������м���H2O2��Ŀ���ǣ� ��Ӧ�����ӷ���ʽΪ ��
��3����֪��
|
�� |
�������↑ʼ����ʱ��pH |
�������������ȫʱ��pH |
|
Fe3�� |
1.9 |
3.2 |
|
Cu2�� |
4.7 |
6.7 |
|
Fe2�� |
7 |
9 |
������е���pH����ѷ�ΧΪ ��
������п������ڵ�����ҺpH���Լ�X�� ��
a��Cu2(OH)2CO3 b��CuO c�� Cu(OH)2 d��NH3•H2O
��4������ܽ��еIJ����� �����ˡ�ϴ�ӡ���� �ڲ������Ҫ�õ���ˮCuCl2����Ҫ�� ����CuCl2��2H2O��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��㶫ʡ�����и�����һ�ε��п��������ۺϻ�ѧ�Ծ��������棩 ���ͣ������
��16�֣�ijͬѧ���ô�����ͭ��������FeO��������������ʣ���ȡ��ˮ�Ȼ�ͭ��������ͼ��

(1)�����������ͭ�����ᷴӦ���������ʽ��___________________��
(2)������м���H2O2��Ŀ��____________________________________��
����II�Ļ�ѧʽΪ_______________________��
(3)��֪��
|
|
�������↑ʼ����ʱ��pH |
�������������ȫʱ��pH |
|
Fe3+ |
1.9 |
3.2 |
|
Cu2+ |
4.7 |
6.7 |
|
Fe2+ |
7 |
9 |
������е���pH����ѷ�ΧΪ____��������ҺpH���Լ�X������______________:
a.NaOH
b.CuO c.Cu(OH)2
d.
(4)����ܵIJ�����___________�����ˡ�ϴ�ӡ����
Ϊ�õ���ˮCuCl2����������ڸ����HCl�����м��� ��ԭ����__________________________________________________________��
��ԭ����__________________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com