
15分) 现用98%浓硫酸(密度1.84g/mL)配制100mL1mol/L的稀硫酸。可供选用的仪器有:
①胶头滴管②烧瓶③烧杯④药匙⑤量筒⑥托盘天平⑦玻璃棒。请回答下列问题:
(1)保存浓硫酸的试剂瓶上除贴B标签外,还需贴上的标签是___________。
(2)配制稀硫酸时,上述仪器中不需要使用的有 (选填序号),还缺少的仪器有 ,使用之前首先必须进行 。
(3)经计算,配制100mL1mol/L的稀硫酸需要用量筒量取上述浓硫酸的体积为 mL。
(4)稀释时,一定要将 沿器壁慢慢倒入 中并不断搅拌.
(5)下列情况对所配制的稀硫酸浓度有何影响?(填写“偏大”、“偏小”、“无影响”)
| A.所用的浓硫酸长时间放置在密封不好的容器中 |
| B.容量瓶用蒸馏水洗涤后残留有少量的水 |
| C.转移溶液后,未洗涤烧杯和玻璃棒就直接定容 |
| D.定容时俯视溶液的凹液面 |
1) D ;(2)②④⑥ ; 100mL容量瓶 ; 检漏 ;(3) 5.4ml ;(4) 浓硫酸 水 (5) A 偏小 B无影响 C偏小 D偏大 E偏小
解析试题分析:(1)浓硫酸是强酸,有很强的腐蚀性,故选D;(2)操作步骤有计算、量取、稀释、洗涤、定容、摇匀等操作,一般用量筒量取浓硫酸,在烧杯中稀释(可用量筒量取水加入烧杯),并用玻璃棒搅拌.冷却后转移到100mL容量瓶中,并用玻璃棒引流,洗涤烧杯、玻璃棒2-3次,并将洗涤液移入容量瓶中,加水至液面距离刻度线1~2cm时,改用胶头滴管滴加,最后定容颠倒摇匀.所以所需仪器有量筒、烧杯、玻璃棒、100mL容量瓶、胶头滴管.故不需要的仪器有②④⑥;还缺少的仪器有100mL容量瓶,而容量瓶等带塞子或活塞的仪器使用前必须查漏,故答案为:②④⑥;100mL容量瓶;查漏;(3)浓硫酸的物质的量浓度为c= =18..4mol/L,设需要的浓硫酸的体积为VmL,根据溶液稀释定律C浓V浓=C稀V稀可知:100mL×1mol/L=18.4mol/L×VmL,解得V=5.4mL,故答案为:5.4;(4)浓硫酸的稀释放热,故应将浓硫酸沿烧杯内壁注入烧杯中的水中,并用玻璃棒不断搅拌,故答案为:浓硫酸;水;(5)A.所用的浓硫酸长时间放置在密封不好的容器中,则浓硫酸的浓度偏小,故配制出的溶液的浓度偏小,故答案为:偏小;B.若容量瓶未干燥即用来配制溶液,对溶液浓度无影响,因为只要定容时正确,至于水是原来就有的还是后来加入的,对浓度无影响,故答案为:无影响;C.转移溶液后,未洗涤烧杯和玻璃棒就直接定容,会造成溶质的损失,则浓度偏小,故答案为:偏小;D.定容时俯视溶液的凹液面,则溶液体积偏小,浓度偏大,故答案为:偏大;E.定容后摇匀,发现液面降低是正常的,又补加少量水,重新达到刻度线则导致浓度偏小,故答案为:偏小.
=18..4mol/L,设需要的浓硫酸的体积为VmL,根据溶液稀释定律C浓V浓=C稀V稀可知:100mL×1mol/L=18.4mol/L×VmL,解得V=5.4mL,故答案为:5.4;(4)浓硫酸的稀释放热,故应将浓硫酸沿烧杯内壁注入烧杯中的水中,并用玻璃棒不断搅拌,故答案为:浓硫酸;水;(5)A.所用的浓硫酸长时间放置在密封不好的容器中,则浓硫酸的浓度偏小,故配制出的溶液的浓度偏小,故答案为:偏小;B.若容量瓶未干燥即用来配制溶液,对溶液浓度无影响,因为只要定容时正确,至于水是原来就有的还是后来加入的,对浓度无影响,故答案为:无影响;C.转移溶液后,未洗涤烧杯和玻璃棒就直接定容,会造成溶质的损失,则浓度偏小,故答案为:偏小;D.定容时俯视溶液的凹液面,则溶液体积偏小,浓度偏大,故答案为:偏大;E.定容后摇匀,发现液面降低是正常的,又补加少量水,重新达到刻度线则导致浓度偏小,故答案为:偏小.
考点:配制一定物质的量浓度的溶液


科目:高中化学 来源: 题型:单选题
为解决全球能源与环境问题,节能减排已成共识。下列措施有利于节能减排的有
| A.举行“地球一小时”熄灯活动 |
| B.露天焚烧稻草和秸秆 |
| C.夏天将空调的温度设置在26℃以上 |
| D.生活垃圾分类回收处理 |
查看答案和解析>>
科目:高中化学 来源: 题型:单选题
化学与社会可持续发展密切相关。下列做法不合理的是
| A.CO2合成可降解的聚碳酸酯类塑料,实现“碳循环” |
| B.提倡步行、骑自行车、乘公交车等“低碳”出行方式 |
| C.将废电池深埋,防止重金属污染 |
| D.开发利用各种新能源,减少对化石燃料的依赖,可以降低空气中PM2.5的含量 |
查看答案和解析>>
科目:高中化学 来源: 题型:实验题
(6分)(1)用5.0mol﹒L-1的NaOH溶液配制0.1mol﹒L-1的NaOH溶液时,如图所示的仪器中,肯定不需要的是 (填序号),配制上述溶液还需要的玻璃仪器
是 (填仪器名称)。
(2)在配制过程中,下列操作将导致所配溶液浓度偏低的是(填编号)
①准确取出的浓NaOH溶液在空气中露置时间过长;
②用量器将浓NaOH溶液直接加入容量瓶,缓慢加入蒸馏水至液面最低点恰好和环形刻度线相切;
③摇匀后,液面低于刻度线,再加蒸馏水至液面最低点恰好和环形刻度线相切;
④稀释NaOH溶液的仪器未洗涤。
查看答案和解析>>
科目:高中化学 来源: 题型:实验题
(12分)实验室需要配制0.1 mol/L NaOH溶液450 mL,回答下列问题:
(1)如图所示的仪器中配制溶液肯定不需要的是 (填序号),
配制上述溶液还需用到的玻璃仪器是 (填仪器名称);
(2)下列操作中,容量瓶所不具备的功能有 (填序号)。
A.配制一定体积准确浓度的标准溶液
B.贮存溶液
C.测量容量瓶规格以下的任意体积的液体
D.用来加热溶解固体溶质
(3)根据计算用托盘天平称取NaOH的质量为 g;
(4)取用任意体积的所配0.1 mol/L NaOH溶液时,下列物理量中不随所取体积的多少而变化的是(填字母) ;
A.溶液中NaOH的物质的量 B.溶液的浓度
C.溶液中Na+的数目 D.溶液的密度
(5)将所配制的NaOH溶液进行测定,发现浓度大于0.1mol/L。请你分析下列哪些操作会引起所配浓度偏大(填写字母) 。
A.烧杯未进行洗涤
B.配制前,容量瓶中有少量蒸馏水
C.NaOH在烧杯中溶解后,未冷却就立即转移到容量瓶中,并进行定容;
D.往容量瓶转移时,有少量液体溅出
E.在容量瓶中定容时俯视容量瓶刻度线
F.定容后塞上瓶塞反复摇匀,静置后,液面不到刻度线,再加水至刻度线。
查看答案和解析>>
科目:高中化学 来源: 题型:实验题
(14分)某同学帮助水质检测站配制800mL 1 mol·L-1NaOH溶液以备使用。
(1)该同学应选择的玻璃仪器除了烧杯、量筒、玻璃棒、胶头滴管外,还有___________。
(2)其操作步骤如下图所示,则如图操作应在下图中的 (填选项字母)之间。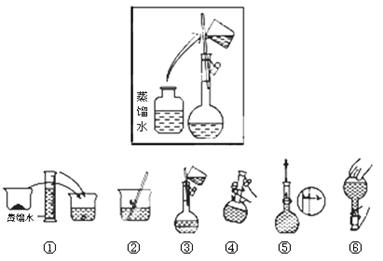
A.②与③ B.①与② C.④与⑤
(3)该同学应用托盘天平称取NaOH固体 g,用质量为33.1 g的烧杯放在托盘天平上称取所需NaOH固体时,请在下图中选出能正确表示游码位置的选项 (填选项字母)。
(4)下列操作对所配溶液的浓度大小有何影响 (填“偏大”、“偏小”或“无影响”)。
①定容时,俯视读数,浓度会 ;
②转移溶液过程中,少量液体溅出来,浓度会 ;
③容量瓶未干燥,浓度会 ;
④定容摇匀后发现溶液凹面低于刻度线,浓度会 。
(5)配制溶液的实际操作过程中,动作要快,否则由于 ,会使配制的NaOH溶液的浓度比1 mol·L-1 (填“大”或“小”)。
查看答案和解析>>
湖北省互联网违法和不良信息举报平台 | 网上有害信息举报专区 | 电信诈骗举报专区 | 涉历史虚无主义有害信息举报专区 | 涉企侵权举报专区
违法和不良信息举报电话:027-86699610 举报邮箱:58377363@163.com