
| ���� | ������ƽ�������룩 | С�ձ� | ����ǯ | ������ | ҩ�� | ��Ͳ |
| ���� |  |  |  |  |  |  |
| ��� | a | b | c | d | e | f |
���� ��1������������Һ���ѡ���������ƿ������m=CVM������Ҫ�������Ƶ�������
��2������һ�������Ĺ�������һ����������ƽ��ҩ�ף�������ʴ��ҩƷӦ��С�ձ�ʢ�ţ�
��3���������������ʵ����ʵ�������Һ�����Ӱ�죬����C=$\frac{n}{V}$������������
��� �⣺��1������475mL0.10mol•L-1��NaOH��Һ��Ӧѡ��500mL����ƿ��ʵ������500mL��Һ����Ҫ������������m=0.5L��0.1mol/L��40g/mol=2.0g��
�ʴ�Ϊ��2.0��
��2���������ƾ��и�ʴ�����Գ����������ƹ���Ӧ�õ�������������ƽ��ҩ�ף�С�ձ���
�ʴ�Ϊ��abe��
����3���ٳ�������ʱ�����̸ߣ����̵ͣ����³����Ĺ�������ƫС�����ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ���ѡ��
�����õ��������⣬���³����Ĺ�������ƫ�����ʵ����ʵ���ƫ����ҺŨ��ƫ�ߣ��ʲ�ѡ��
����Һת�Ƶ�����ƿ��δ����ϴ�Ӳ��������²���������ģ����ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ���ѡ��
��ת����Һǰ����ƿ������������ˮ�������ʵ����ʵ�������Һ�����������Ӱ�죬��ҺŨ�Ȳ��䣬�ʲ�ѡ��
�ݶ���ʱ����������ƿ�Ŀ̶��ߣ�������Һ���ƫ����ҺŨ��ƫ�ͣ���ѡ��
�����ձ����ܽ�NaOH��������������Һע������ƿ�У���ȴ����Һ���ƫС����ҺŨ��ƫ�ߣ��ʲ�ѡ��
�߶��ݺ�ҡ�ȣ�����Һ�潵�ͣ��ֲ�������ˮ�����´ﵽ�̶��ߣ�������Һ���ƫ����ҺŨ��ƫ�ͣ���ѡ��
��ѡ���٢ۢݢߣ�
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƣ���ȷ����ԭ�������������ǽ���ؼ���ע�������ʴ��ҩƷӦ��С�ձ�ʢ�ţ�ע���������ķ�����


 99��1������ĩ��ѵ��ϵ�д�
99��1������ĩ��ѵ��ϵ�д� ��ǿ��У��ĩ���100��ϵ�д�
��ǿ��У��ĩ���100��ϵ�д� �óɼ�1��1��ĩ���100��ϵ�д�
�óɼ�1��1��ĩ���100��ϵ�д� ��״Ԫ���źþ�ϵ�д�
��״Ԫ���źþ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ��� | �� | �� | �� | �� |
| ��Һ | NaCl | CH3COONH4 | NaHCO3 | 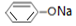 |
| pH | 7.0 | 7.0 | 8.4 | 9.9 |
| A�� | ���ԣ�H2CO3��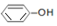 | |
| B�� | ˮ���������c��H+������=�� | |
| C�� | ��Һ���У�c��HCO3-��+c��CO32-��+c��H2CO3��=0.1mol•L-1 | |
| D�� | ��Һ���У�c��Na+����c��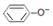 ����c��OH-����c��H+�� ����c��OH-����c��H+�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ���� | ��; | |
| A | HF��Һ�������� | ����HF��Һ�ڲ����Ͽ�ͼ�� |
| B | SO2����ǿ��ԭ�� | SO2������Ư�ոѡ�֯�� |
| C | CuSO4���������� | CuSO4����ʯ���䲨����Һ |
| D | CH3CH2OHȼ�շų��������� | �ƾ����������Դ |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | c��NH4+����c��Cl-����c��OH- ����c�� H+ �� | B�� | c��Cl-����c��NH4+����c�� H+ ����c��OH-�� | ||
| C�� | c��NH4+��+c�� H+ ��=c��Cl-��+c��OH- �� | D�� | c��NH4+��+c�� NH3•H2O ��=c��Cl-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ñ�ˮ�������ȴSO3���壻����I2 | |
| B�� | ľ̿����NO2���壻��Cl2ͨ��Ʒ����Һ�� | |
| C�� | ��ʢ��NO�ļ���ƿ����ȴNO2���� | |
| D�� | ��Ʒ����Һ��ͨ��SO2����FeCl3��Һ�μ�KSCN��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ö��Ե缫��������Ȼ��ƣ�2Cl-+2H2O�TCl2��+H2��+2OH- | |
| B�� | ��ʳ�׳�ȥˮƿ�е�ˮ����CO32-+2CH3COOH�T2CH3COO-+CO2��+H2O | |
| C�� | ��ϡ����������Һ���ն���������2OH-+2NO2�TNO3-+NO��+H2O | |
| D�� | ������������Һ��ȥ�����������Ĥ��Al2O+2OH-�T2AlO2-+H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
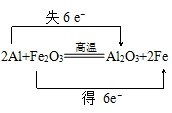 ��
���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com