
ЃЈ20ЗжЃЉгУ18.4molЁЄL-1ЕФХЈСђЫсЃЌХфжЦ 100 ml 1.0 molЁЄL-1ЕФЯЁСђЫсЃЌЧыЛиД№вдЯТЮЪЬтЃК
ЃЈ1ЃЉашвЊ18.4molЁЄL-1ЕФХЈСђЫс mlЁЃ
ЃЈ2ЃЉЯТСаФФзщвЧЦїдкХфжЦЪБВЛашвЊгУЕН ЃЈ ЃЉ
ЂйЭаХЬЬьЦН ЂкЗжвКТЉЖЗ Ђл250mlШнСПЦП ЂмЩеБ ЂнНКЭЗЕЮЙм
ЂоСПЭВ ЂпВЃСЇАєЂрЬњМмЬЈЃЈДјЬњМаЃЉ Ђс100mlШнСПЦП
AЃЎЂлЂмЂнЂпЂс BЃЎЂйЂкЂнЂоЂр CЃЎЂйЂкЂлЂр DЃЎЂлЂмЂнЂо
ЃЈ3ЃЉЯТСаЪЕбщВНжшжаЃЌе§ШЗЕФВйзїЫГађгІИУЪЧЃК
A гУСПЭВСПШЁХЈСђЫсЃЌЛКЛКЕЙШызАгадМ50mlеєСѓЫЎЕФЩеБРяЃЌВЂгУВЃСЇАєНСАшЁЃ
B гУдМ30mlеєСѓЫЎЃЌЗжГіШ§ДЮЯДЕгЩеБКЭВЃСЇАєЃЌНЋУПДЮЯДЕгвКЖМЕЙШыШнСПЦПжаЃЛ
C НЋЯЁЪЭКѓЕФСђЫсаЁаФЕиЕЙШыШнСПЦПжаЃЛ
D.МьВщ100mlШнСПЦПЦППкЪЧЗёгаТЉвКЯжЯѓЃЛ
E.НЋеєСѓЫЎжБНгМгШыШнСПЦПЃЌжСвКУцНгНќПЬЖШЯп1ЁЊЁЊ2cmДІЃЛ
F.ИЧНєЦПШћЃЌЗДИДЕпЕЙеёЕДЃЌвЁдШШмвКЃЛ
G.гУНКЭЗЕЮЙмЯђШнСПЦПРяж№ЕЮМгШыеєСѓЫЎЃЌжСвКУцзюЕЭЕугыПЬЖШЯпЯрЧаЃЛ
ЃЈ4ЃЉНјааAВНжшВйзїЕФЪБКђЃЌгІИУбЁгУ
Ђй10 mlСПЭВ Ђк50 mlСПЭВ Ђл5000 mlСПЭВ Ђм1000 mlСПЭВ
ЃЈ5ЃЉНјааAВНВйзїКѓЃЌБиаы КѓЃЌВХФмНјааCВНВйзїЁЃ
ЃЈ1ЃЉ5.4
ЃЈ2ЃЉC
ЃЈ3ЃЉD A C B E G F
ЃЈ4ЃЉ1
ЃЈ5ЃЉЕШШмвКРфШДжСЪвЮТ
НтЮіЪдЬтЗжЮіЃКЃЈ1ЃЉЂйУмЖШІб=1.84g?mL-1ЃЌжЪСПЗжЪ§ЮЊ98%ЕФХЈСђЫсЕФХЈЖШЮЊЃКc=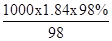 =18.4mol/L
=18.4mol/L
ХфжЦ100mL1mol?L-1ЕФЯЁСђЫсЃЌашвЊИУХЈСђЫсЕФЬхЛ§ЮЊЃК Ёж0.0054L=5.4mlЃЌД№АИЃК5.4ЃЛ
Ёж0.0054L=5.4mlЃЌД№АИЃК5.4ЃЛ
ЃЈ2ЃЉХфжЦ100mL1mol/LЕФЯЁСђЫсЕФВНжшЮЊЃКМЦЫуЁњСПШЁЁњЯЁЪЭЁЂРфШДЁњвЦвКЁњЖЈШнЁњвЁдШЁњзАЦПЁњЬљ
ЧЉЃЌашвЊЪЙгУЕФвЧЦїгаЃКЩеБ ЁЂСПЭВ ЁЂ100mLШнСПЦПЁЂВЃСЇАєЁЂЖЈШнЪББиаыЪЙгУНКЭЗЕЮЙмЃЌЫљвдЛЙШБ
ЩйЕФвЧЦїЮЊНКЭЗЕЮЙмЃЌВЛвЊгУЕНЕФЪЧЂйЂкЂлЂрЃЌД№АИбЁCЃЛ
ЪЕбщЙ§ГЬжаЯШМьВщШнСПЦПЪЧЗёТЉвКЃЌШЛКѓИљОнМЦЫуЁњСПШЁЁњЯЁЪЭЁЂРфШДЁњвЦвКЁњЖЈШнЁњвЁдШЁњзА
ЦПЁњЬљЧЉЕУВНжшХфжУШмвКЃЌД№АИЮЊDACGEBFЃЛ
ЭЈЙ§МЦЫуашвЊХЈСђЫс5.4mlЃЌИљОнбЁШЁСПЭВЁАДѓЖјНќЁБЕФддђЃЌгІбЁгУ10mlЕФСПЭВЃЌД№АИбЁЂйЃЛ
ЃЈ5ЃЉШєЮДРфШДЕНЪвЮТМДЕЙШыШнСПЦПжаЃЌЛсЪЙХфжУСђЫсЕФХЈЖШЦЋДѓЃЌД№АИЕШШмвКРфШДЕНЪвЮТЃЛД№АИЃКЕШ
ШмвКРфШДжСЪвЮТ


 ЪРМЭАйЭЈЦкФЉН№ОэЯЕСаД№АИ
ЪРМЭАйЭЈЦкФЉН№ОэЯЕСаД№АИ
| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃКЕЅбЁЬт
ЛЏбЇгыЩчЛсПЩГжајЗЂеЙУмЧаЯрЙиЁЃЯТСазіЗЈВЛКЯРэЕФЪЧ
| AЃЎCO2КЯГЩПЩНЕНтЕФОлЬМЫсѕЅРрЫмСЯЃЌЪЕЯжЁАЬМбЛЗЁБ |
| BЃЎЬсГЋВНааЁЂЦяздааГЕЁЂГЫЙЋНЛГЕЕШЁАЕЭЬМЁБГіааЗНЪН |
| CЃЎНЋЗЯЕчГиЩюТёЃЌЗРжЙжиН№ЪєЮлШО |
| DЃЎПЊЗЂРћгУИїжжаТФмдДЃЌМѕЩйЖдЛЏЪЏШМСЯЕФвРРЕЃЌПЩвдНЕЕЭПеЦјжаPM2.5ЕФКЌСП |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃКЪЕбщЬт
ЃЈ17ЗжЃЉПЦбЇЬНОП
ФГбЇЯАаЁзщИљОнЭМжаЫљЪОЕФЕчНтЫЎдРэНјааЪЕбщЃЌВЂЖдЪЕбщЕУЕНЕФгыВщдФзЪСЯЛёЕУЕФЪ§ОнНјааДІРэЁЂЗжЮіЃЌЧыгыЫћУЧвЛЦ№НјааЬНОПЁЃЧыАДвЊЧѓЬюаДЁЃ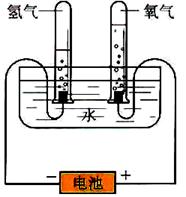
ЃЈ1ЃЉЙлВьВЛЭЌЪБМфЪдЙмФкЕФH2КЭO2ЬхЛ§ЕФБШжЕОљдМЮЊ ЁЃ
ЃЈ2ЃЉМйЩшЕчНтСЫ1.8g H2OЃЌИљОнЕчНтЫЎЕФЛЏбЇЗНГЬЪНМЦЫуЩњГЩH2ЁЂO2ЕФжЪСПЃЌМЦЫуЙ§ГЬШчЯТЃК
ВЂЬюБэШчЯТЃК
| | жЪСПg | ЮяжЪЕФСПmol | H2КЭO2ЮяжЪЕФСПЕФБШ |
| H2 | | | |
| O2 | | |
| ЬѕМў | ЮяжЪ | 1 molЮяжЪЕФЬхЛ§ |
| 0Ёц101kPa | H2 | 22.3 L |
| O2 | 22.4 L | |
| CO2 | 22.4 L | |
| 25Ёц101kPa | H2 | 24.4L |
| O2 | 24.5L | |
| CO2 | 24.5L |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃКЪЕбщЬт
ЃЈУППе1ЗжЃЌЙВ8ЗжЃЉЪЕбщЪвашвЊХфжЦ0.1 molЁЄLЃ1CuSO4ШмвК480 mLЁЃ
АДЯТСаВйзїВНжшЬюЩЯЪЪЕБЕФЮФзжЃЌвдЪЙећИіВйзїЭъећЁЃ
ЃЈ1ЃЉбЁдёвЧЦїЁЃЭъГЩБОЪЕбщЫљБиашЕФвЧЦїгаЃКЭаХЬЬьЦН(ОЋШЗЕН0.1 g)ЁЂвЉГзЁЂЩеБЁЂВЃСЇАєЁЂ______ЁЂ________вдМАЕШжЪСПЕФСНЦЌТЫжНЁЃ
ЃЈ2ЃЉМЦЫуЃЌгІбЁдёЯТСае§ШЗ________
| AЃЎашвЊCuSO4 ЙЬЬх8g | BЃЎашвЊCuSO4ЁЄ5H2OОЇЬх12.0 g |
| CЃЎашвЊCuSO4ЁЄ5H2OОЇЬх12.5 g | DЃЎашвЊCuSO4ЙЬЬх7.7 g |

ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃКЪЕбщЬт
гУNa2CO3ЁЄ10H2OОЇЬхЃЌХфжЦ0ЃЎ2 molЁЄL-1ЕФNa2CO3ШмвК480 mLЁЃ
ЃЈ1ЃЉгІГЦШЁNa2CO3ЁЄ10H2OОЇЬхЕФжЪСПЮЊЁЁЁЁЁЁЁЁЁЃ
ЃЈ2ЃЉИљОнЯТСаВйзїЖдЫљХфШмвКЕФХЈЖШВњЩњЕФгАЯьЃЌЭъГЩЯТСавЊЧѓЃК
ЂйNa2CO3ЁЄ10H2OОЇЬхЪЇШЅСЫВПЗжНсОЇЫЎ
ЂкЬМЫсФЦОЇЬхВЛДПЃЌЦфжаЛьгаТШЛЏФЦ
ЂлГЦСПЬМЫсФЦОЇЬхЪБЫљгУэРТыЩњат
ЂмШнСПЦПЮДОИЩдяЪЙгУ
Цфжав§Ц№ЫљХфШмвКХЈЖШЦЋИпЕФгаЁЁЁЁЁЁЁЁ(ЬюађКХЃЌЯТЭЌ)ЃЌЦЋЕЭЕФгаЁЁЁЁЁЁЁЁЃЌЮогАЯьЕФгаЁЁЁЁЁЁЁЁЁЃ
ЃЈ3ЃЉФГЭЌбЇИФгУЙЬЬхNa2CO3ХфжЦЩЯЪіNa2CO3ШмвКЕФЙ§ГЬШчЭМЫљЪОЃК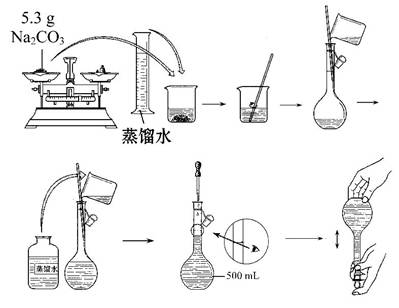
ФуШЯЮЊИУЭЌбЇЕФДэЮѓВНжшгаЁЁЁЁЁЁЁЁ(ЬюађКХ)ЁЃ
| AЃЎ1ДІ | BЃЎ2ДІ | CЃЎ3ДІ | DЃЎ4ДІ |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃКЪЕбщЬт
ЃЈ13ЗжЃЉвбжЊHIЪЧвЛжжЮоЩЋЃЌгаДЬМЄадЦјЮЖЃЌМЋвзШмгкЫЎЕФЦјЬхЃЌHIЕФЫЎШмвКГЦжЎЮЊЧтЕтЫсЃЌЪЧвЛжжЧПЫсЁЃ
ЃЈ1ЃЉЧыаДГіЕтдЊЫидкжмЦкБэЕФЮЛжУЃКЕкЮхжмЦк зхЁЃ
ЃЈ2ЃЉНЋHIЦјЬхЭЈШывЛЖЈСПЕФХЈСђЫсжаЃЌВњЩњЕФЛьКЯЦјЬхГ§КЌHIЁЂЩйСПЕФI2еєЦјКЭЫЎеєЦјЭтЃЌЛЙПЩФм
га ЦјЬхЁЃ
ЃЈ3ЃЉаЁУїФтЖдHIЭЈШыХЈСђЫсКѓЕФЛьКЯЦјЬхГЩЗжНјаабщжЄКЭЬНОПЁЃЫћЩшМЦСЫШчгвЪЕбщзАжУЭМГѕВНЬНОПЩшМЦЃК
ЂйЦфжажБаЮВЃСЇЙмжаЫљзАЕФвЉЦЗЪЧ ЃЈаДУћГЦЃЉ
ЂкзуСПЫФТШЛЏЬМЕФСНИізїгУЪЧЃК ЃЌ ЁЃ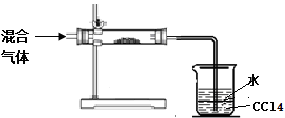
НјвЛВНЬНОПЃК
ВщдФзЪСЯЃКбѕЛЏадЧПШѕKMnO4>HNO3>I2>SO42ЃЃЌЧвНЯЯЁЕФЯѕЫсВЛФмбѕЛЏI2ЁЃ
ЂлаЁУїФтЖдШмдкЫЎВуЕФЦфЫќЛьКЯЦјЬхГЩЗжзіНјвЛВНЬНОПЁЃЧыФуДгвдЯТЯобЁЪдМСжабЁдёКЯЪЪЕФЪдМСАяаЁУїЭъГЩЪЕбщБЈИцЁЃ
ЯобЁЕФЪдМСЃКЪЏШяЪдМСЁЂЦЗКьШмвКЁЂЫсадKMnO4ШмвКЁЂ0.1mol/L HNO3ЁЂЕэЗлШмвКЁЂBaCl2ШмвК
| ЪЕбщЗНАИ | ПЩФмЕФЯжЯѓКЭЯргІЕФНсТл |
| ШЁЩйСПЩеБжаЕФЩЯВуШмвКЗжзАШыAЁЂBСНжЇЪдЙмжа | |
| | |
| | |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃКЕЅбЁЬт
ЯТСаЮяжЪЕФЗжРыЗНЗЈВЛе§ШЗЕФЪЧ
| AЃЎгУЙ§ТЫЕФЗНЗЈГ§ШЅЪГбЮжаФрЩГ |
| BЃЎгУеєСѓЕФЗНЗЈНЋКЃЫЎЕЛЏ |
| CЃЎгУОЦОЋнЭШЁЕтЫЎжаЕФЕтЕЅжЪ |
| DЃЎгУМгШШЕФЗНЗЈГ§ШЅЬМЫсФЦжаЕФЬМЫсЧтФЦ |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃКЭЦЖЯЬт
ЃЈ9ЗжЃЉМзЁЂБћЁЂЖЁЪЧгЩЖЬжмЦкдЊЫизщГЩЕФЮяжЪЃЌЫќУЧжЎМфДцдкШчЯТзЊЛЏЙиЯЕЁЃ
Мз + H2O Ёњ Бћ + ЖЁ
ЃЈ1ЃЉзЊЛЏЙиЯЕжаЫљЩцМАЕФЗДгІЮЊЗЧбѕЛЏЛЙдЗДгІЁЃ
ЂйШєМзЪЧвЛжжФЦбЮЃЌЖЁЮЊСНадЧтбѕЛЏЮяЃЌдђМзЕФЛЏбЇЪНЮЊ ЃЌЖЁЕФЫсЪНЕчРыЗНГЬЪНЮЊ ЁЃ
ЂкШєМзЪЧгЩNКЭClдЊЫизщГЩЕФЛЏКЯЮяЃЌЦфЗжзгНсЙЙФЃаЭШчЭМЫљЪОЃЌБћОпгаЦЏАзадЁЃдђМзжаClдЊЫиЕФЛЏКЯМлЪЧ ЃЌЖЁгыH2OгаЯрЭЌЕФЕчзгзмЪ§ЃЌдђЖЁЕФЕчзгЪНЮЊ 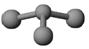
ЃЈ2ЃЉзЊЛЏЙиЯЕжаЫљЩцМАЕФЗДгІЮЊбѕЛЏЛЙдЗДгІЁЃ
ЂйШєМзКЭБћЪЧЭЌжїзхдЊЫизщГЩЕФЕЅжЪЃЌЧвзщГЩМзЕФдЊЫиЮЛгкЕкШ§жмЦкЃЌДЫЗДгІЕФРызгЗНГЬЪНЪЧ ЁЃ
ЂкШєБћКЭЖЁЖМПЩдквЛЖЈЬѕМўЯТЛЙдCuOЃЌДЫЗДгІЕФЛЏбЇЗНГЬЪНЪЧ ЁЃ
ВщПДД№АИКЭНтЮі>>
АйЖШжТаХ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com