
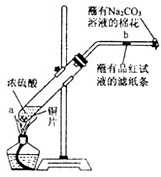
��12�֣�ijѧ����Ũ�������ʵ�ʵ�飺��һ֧�Թ��з���һ���С��ͭƬ���ټ���2mLŨ���ᣬȻ����Թ̶ܹ�������̨�ϡ���һС��պ��Ʒ����Һ����ֽ������е�����Ƥ���IJ������С������Թܿڣ��ڲ����ܿڴ�����һ��պ��Na2CO3��Һ�����������Թܣ��۲�����.�ش��������⣺

��1��д���Թ��з�����Ӧ�Ļ�ѧ����ʽ ��
��2���Թ��е�Һ�巴Ӧһ��ʱ���b����ֽ���ı仯Ϊ �����Թ��з�Ӧֹͣ�������ܷ���պ��Ʒ����Һ����ֽ�����ȣ���ֽ���ı仯Ϊ ��
��3��պ��Na2CO3��Һ������������ ��
��4��������������γɹ��̿������з�Ӧ�е� ����ʾ��

��5��Ũ������������Ҫ���ʣ����뺬��ˮ�ֵ��������ù����в�����ʾ��������
A������ B����ˮ�� C��ǿ������ D����ˮ��
��12�֣���1��Cu+2H2SO4(Ũ)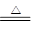 CuSO4+ SO2��+2H2O
CuSO4+ SO2��+2H2O
��2��պ��Ʒ����Һ����ֽ��ɫ ����ֽ���
��3�����ն���SO2����ֹ��Ⱦ���� ��4��A B ��5��A
��������
�����������1���ڼ��ȵ������£�Ũ�����ͭ��Ӧ�Ļ�ѧ����ʽ��Cu+2H2SO4(Ũ)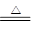 CuSO4+ SO2��+2H2O��
CuSO4+ SO2��+2H2O��
��2������Ļ�ԭ����SO2����Ư���ԣ���ʹƷ����Һ��ɫ����SO2��Ư���Dz��ȶ��ģ��ڼ��ȵ�����£����Իָ�����������ɫ��������ֽ���ָֻ���ɫ��
��3��SO2�Ǵ�����Ⱦ����պ��Na2CO3��Һ���������������ն���SO2����ֹ��Ⱦ������
��4�������е�SO2����������SO3��SO3����ˮ�������ᣬ���γ����꣬��ѡAB��
��5��Ũ���������ˮ�ԡ���ˮ�Ժ�ǿ�����ԡ�����ʹ����̿����������������CO2��SO2��ˮ�������ڸñ仯�����У�û��������������ԣ���ѡA��
���㣺����Ũ��������ʡ�SO2�ļ����β������
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣���������ǿ���������У������ڿ���ѧ��������Ҳ����������ѧ����ʵ��̽���������Լ�������������ͬʱҲ����������ѧ���Ļ���������ʶ����ǿѧ����������θС�


 ���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�
���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д� ����ѵ��ϵ�д�
����ѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ijѧ����Ũ�������ʵ�ʵ�飺
ijѧ����Ũ�������ʵ�ʵ�飺
| ||
| ||
| ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�



�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ijѧ����Ũ�������ʵ�ʵ�飺
ijѧ����Ũ�������ʵ�ʵ�飺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ���㽭ʡ��ɽ���е���У��һ��ѧ����ĩ������ѧ�Ծ����������� ���ͣ������
��12�֣�ijѧ����Ũ�������ʵ�ʵ�飺��һ֧�Թ��з���һ���С��ͭƬ���ټ���2mLŨ���ᣬȻ����Թ̶ܹ�������̨�ϡ���һС��պ��Ʒ����Һ����ֽ������е�����Ƥ���IJ������С������Թܿڣ��ڲ����ܿڴ�����һ��պ��Na2CO3��Һ�����������Թܣ��۲�����.�ش��������⣺
��1��д���Թ��з�����Ӧ�Ļ�ѧ����ʽ ��
��2���Թ��е�Һ�巴Ӧһ��ʱ���b����ֽ���ı仯Ϊ �����Թ��з�Ӧֹͣ�������ܷ���պ��Ʒ����Һ����ֽ�����ȣ���ֽ���ı仯Ϊ ��
��3��պ��Na2CO3��Һ������������ ��
��4��������������γɹ��̿������з�Ӧ�е� ����ʾ��
��5��Ũ������������Ҫ���ʣ����뺬��ˮ�ֵ��������ù����в�����ʾ��������
| A������ | B����ˮ�� | C��ǿ������ | D����ˮ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com