
ʵ�����Ʊ������飨C2H5Br����װ�úͲ�����ͼ������֪������ķе�38.4�棩
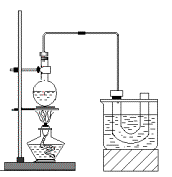
�ټ��װ�õ������ԣ���װ��ͼ��ʾ��U�ܺʹ��ձ��м����ˮ��
����Բ����ƿ�м���10mL95%�Ҵ���28mLŨ���ᣬȻ�������ϸ��13g�廯�ƺͼ������Ƭ��
��С����ȣ�ʹ���ַ�Ӧ��
�ش��������⣺
��1����ʵ����ȡ������Ļ�ѧ����ʽΪ�����ɵ���ΪNaHSO4����______________________��
��2����Ӧʱ���¶ȹ��ߣ��ɿ����к���ɫ��������������廯ѧʽΪ__________��
��3��Ϊ�˸��õĿ��Ʒ�Ӧ�¶ȣ�����ͼʾ��С����ȣ����õļ��ȷ�ʽ��__________��
��4��U���ڿɹ۲쵽��������_____________________________��
��5����Ӧ������U�ι��д��Ƶ�C2H5Br���ػ�ɫ��Ϊ�˳�ȥ�ֲ�Ʒ�е����ʣ���ѡ�������Լ��е�_________________������ţ�
a���� b��H2O c��Na2SO3��Һ d��CCl4
�������Ҫ����������______________�����������ƣ���
��6�����м���ʵ�鲽�裬�����ڼ�������������Ԫ�أ�����ȷ�IJ���˳���ǣ�ȡ���������飬Ȼ��__________________������ţ���
�ټ��ȣ��ڼ���AgNO3��Һ���ۼ���ϡHNO3�ữ���ܼ���NaOH��Һ������ȴ
��11�֣���1��C2H5OH+NaBr+H2SO4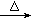 NaHSO4+C2H5Br+H2O
��2�֣�
NaHSO4+C2H5Br+H2O
��2�֣�
��2��Br2������SO2���۷� ����2�֣�
��3��ˮԡ���� ��1�֣�
��4������״Һ�����ɣ�1�֣�
��5��c��2�֣� ��Һ©����1�֣���
��6���ܢ٢ݢۢڣ�2�֣�˳����÷֣�
��������
�����������1���������֪��C4H9OH������ͬ���칹�壬C2H5OH+NaBr+H2SO4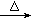 NaHSO4+C2H5Br+H2O����2�����¶ȹ��ߣ�Ũ���ὫNaBr����Ϊ����ɫ��Br2����3��ˮԡ�����ܿ��Ƽ����¶ȣ���4��������ķе�38.4�棬��ˮԡ�ɵõ���ɫ��״Һ�壻��5��Br2������C2H5Br������CCl4������ˮ�����Ա�Na2SO3��Һ��ԭΪBr������ѡc����ַ�Ӧ���Һ���÷�Һ©�����룻��6��±������ǿ����Һ�м���ʱˮ�⣬��ȴ���ϡ�����ữ��������������Һ�ɼ��������ӵĴ��ڣ��Ӷ��ж���Ԫ�صĴ��ڡ�
NaHSO4+C2H5Br+H2O����2�����¶ȹ��ߣ�Ũ���ὫNaBr����Ϊ����ɫ��Br2����3��ˮԡ�����ܿ��Ƽ����¶ȣ���4��������ķе�38.4�棬��ˮԡ�ɵõ���ɫ��״Һ�壻��5��Br2������C2H5Br������CCl4������ˮ�����Ա�Na2SO3��Һ��ԭΪBr������ѡc����ַ�Ӧ���Һ���÷�Һ©�����룻��6��±������ǿ����Һ�м���ʱˮ�⣬��ȴ���ϡ�����ữ��������������Һ�ɼ��������ӵĴ��ڣ��Ӷ��ж���Ԫ�صĴ��ڡ�
���㣺���⿼����������Ʊ�������Ӧ����Ӧ�����Ŀ��ơ�ʵ���������ķ�����ᴿ�������������е���Ԫ�صȡ�


 ��ĩ��ϰ���ϵ�д�
��ĩ��ϰ���ϵ�д� ����ѧ�䵥Ԫ������ĩר����100��ϵ�д�
����ѧ�䵥Ԫ������ĩר����100��ϵ�д� �Ƹ�360�ȶ����ܾ�ϵ�д�
�Ƹ�360�ȶ����ܾ�ϵ�д� ���⿼����Ԫ���Ծ�ϵ�д�
���⿼����Ԫ���Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
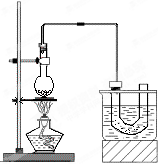 ��ʵ�����Ʊ������飨C2H5Br����װ�úͲ�����ͼ������֪������ķе�38.4�棩
��ʵ�����Ʊ������飨C2H5Br����װ�úͲ�����ͼ������֪������ķе�38.4�棩| �� |
| �� |
| �� |
| �� |
| �� |
| �� |
| �� |
| �� |
| �� |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��֪��CH3CH2OH+NaBr+H2SO4��Ũ��
��֪��CH3CH2OH+NaBr+H2SO4��Ũ��| �� |
| �� |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ������ķе���38.4�棬�ܶ���1.46g/cm3����ͼΪʵ�����Ʊ��������װ��ʾ��ͼ���г���������ȥ��������G��ʢ����ˮ��ʵ��ʱѡ�õ�ҩƷ�У��廯�ơ�95%�Ҵ���ŨH2SO4���Ʊ������б߷�Ӧ��������������������ˮ���ռ�����ã�
������ķе���38.4�棬�ܶ���1.46g/cm3����ͼΪʵ�����Ʊ��������װ��ʾ��ͼ���г���������ȥ��������G��ʢ����ˮ��ʵ��ʱѡ�õ�ҩƷ�У��廯�ơ�95%�Ҵ���ŨH2SO4���Ʊ������б߷�Ӧ��������������������ˮ���ռ�����ã��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| �Ҵ� | ������ | ������ | 1-�嶡�� | |
| �ܶ�/g?cm-3 | 0.7893 | 1.4604 | 0.8098 | 1.2758 |
| �е�/�� | 78.5 | 38.4 | 117.2 | 101.6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �Ҵ� | ������ | ������ | 1-�嶡�� | |
| �ܶ�/g?cm-3 | 0.7893 | 1.4604 | 0.8098 | 1.2758 |
| �е�/�� | 78.5 | 38.4 | 117.2 | 101.6 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com