
 ������ĩ��ϰ��ѵ��ϵ�д�
������ĩ��ϰ��ѵ��ϵ�д� С��ʿ��ĩ����100��ϵ�д�
С��ʿ��ĩ����100��ϵ�д� ��У��ʦ������ҵ���Ӻ����Ծ�ϵ�д�
��У��ʦ������ҵ���Ӻ����Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
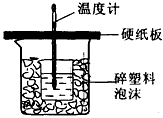
 ��50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺
��50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
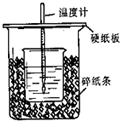 ��50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺
��50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
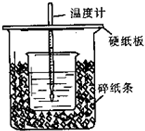 ��50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺
��50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺| �¶� ��� |
��ʼ�¶�t1/�� | ��ֹ�¶� T2/�� |
�¶Ȳ� ��t/�� | ||
| HCl | NaOH | ƽ��ֵ | |||
| 1 | 25 | 25 | 27.3 | ||
| 2 | 25 | 25 | 27.4 | ||
| 3 | 25 | 25 | 28.6 | ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�긣��ʡ�����и߶���ѧ��10���¿����ƻ�ѧ�� ���ͣ������
��7�֣��к�����ָ�������кͷ�Ӧ����lmol H2O���ų���������ijѧ����ͨ���ⶨ��Ӧ���������ų��������������к��ȡ�����50mL0.5mol/L��������50mL0.55mol/L��NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ����ش��������⣺
(1) ��ʵ��װ���Ͽ���ͼ����ȱ�ٵ�һ�ֲ�����Ʒ��__________________���ձ���������������ĭ��������______________ ___________________��
(2) ���ձ����粻��Ӳֽ�壬����õ��к�����ֵ_______(�ƫ����ƫС��������Ӱ�족)
(3) ʵ���и���60 mL 0.50 mol/L�������50mL 0.55 mol/L��NaOH��Һ���з�Ӧ��
������ʵ����ȣ����ų�������________(���ȡ�����ȡ�)��������____________
___________________________________�������к��ȵ���ֵ��________(���ȡ�
����ȡ�)��������__________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�������ʡ��һ��ѧ�����п��Ի�ѧ�Ծ� ���ͣ�ʵ����
�к�����ָ�������кͷ�Ӧ����lmol H2O���ų���������ijѧ����ͨ���ⶨ��Ӧ���������ų��������������к��ȡ�����50mL0.5mol/L��������50mL0.55mol/L��NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ����ش��������⣺
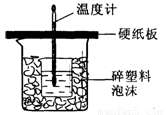
(1) ��ʵ��װ���Ͽ���ͼ����ȱ�ٵ�һ�ֲ�����Ʒ��__________________����װ�õIJ���֮���� �������Ը����������ֵ��
����ƫ��ƫС����Ӱ�죩��
(2) ʵ���и���60 mL 0.50 mol/L�������50mL 0.55 mol/L��NaOH��Һ���з�Ӧ��������ʵ����ȣ����ų�������________(���ȡ�����ȡ�)�������к��ȵ���ֵ��________(���ȡ�����ȡ�)��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com