
ijͬѧ��̽��ʳƷ���Ӽ������NH4Al(SO4)2��12H2O���·ֽ�������
��1��Ԥ�������й�����������Ԥ������������ ��
A��NH3��N2��SO2��H2O B��NH3��SO3��H2O
C��NH3��SO2��H2O D��NH3��N2��SO3��SO2��H2O
��2�����Լ��飺ȡһ������������������ʵ��̽�����
�ٰ�ͼʾ��װ���������ȼ������װ�õ������ԣ������� ��
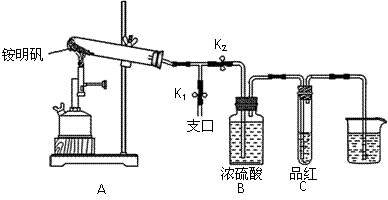 |
�ڼ�סֹˮ��K1����ֹˮ��K2���þƾ���Ƴ�����ա�ʵ������У�װ��A�͵�����δ������ɫ���壻�Թ�C�е�Ʒ����Һ��ɫ����֧�ڴ��ɼ��鵽NH3�������� ����װ��A��B֮���T�͵����г��ְ�ɫ���壬�ð�ɫ��������� ������һ�����ʵĻ�ѧʽ����
�۷����ó�װ��A�Թ��в����İ�ɫ���������������д��������NaOH��Һ�����ӷ���ʽ ��
��Ϊ�˷�ֹ������ʵ�����ʱ������ ������ĸ��ţ���Ȼ��Ϩ��ƾ���ơ�
A��ȡ���ձ��еĵ��� B����ֹˮ��K1 C���ر�ֹˮ��K2
��3�������ͽ��ۣ�ʵ��֤����������ǣ�1��D�е�5�����塣��ͬ�����²������N2��SO2��������Ƕ�ֵ��V��N2����V��SO2��= ��
��16�֣���1��C��2�֣�
��2���ٹر�֧�ڿ���K1����K2��1�֣��������ĵ���ͨ��ˮ�У��ȴ��Թܣ�1�֣����������ӵ����г������ݣ�1�֣�����ֹͣ���Ⱥ��ڵ���������һ��ˮ������1�֣���֤�������Ժá�����4�֣�
�ڴ�K1����պ��Ũ����IJ���������֧�ڣ������ְ��̣����߲����Լ���������Ҳ���֣���2�֣�������ʪ����ɫʯ����ֽ���鲻�÷֣���(NH4)2SO4����(NH4)2SO3����SO3(��ʽ�μ��������ʵĻ����Ҳ����)��2�֣�
��Al2O3 +2OH-=2AlO2-+H2O��Al2O3 +3H2O +2OH-=2Al(OH)4-��2�֣���ƽ�����0�֣�
��B��C����BC��2�֣�
��3��1:3��2�֣�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ȤС�����������ʵ��װ�á�ʵ��ʱ,�ȶϿ�K2,�պ�K1,�����������ݲ���:һ��ʱ���,�Ͽ�K1,�պ�K2,���ֵ�����Aָ��ƫת�������й�������ȷ����(����)

A.�Ͽ�K2,�պ�K1ʱ,�ܷ�Ӧ�����ӷ���ʽΪ:2H++2Cl- Cl2��+H2��
Cl2��+H2��
B.�Ͽ�K2,�պ�K1ʱ,ʯī�缫������Һ���
C. �Ͽ�K1,�պ�K2ʱ,ʯī�缫������
D. �Ͽ�K1,�պ�K2ʱ,ͭ�缫�ϵĵ缫��ӦΪ:Cl2+2e- 2Cl-
2Cl-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й����Ȼ�ѧ��Ӧ����������ȷ���� ( )
A��HCl��NaOH��Ӧ���к��Ȧ�H����57.3kJ/mol����H2SO4��Ca(OH)2��Ӧ���к���
��H��2��(��57.3)kJ/mol
B��CO(g)��ȼ������283.0kJ/mol����2CO2(g) ��2CO(g)��O2(g)��Ӧ��
��H��+2��283.0kJ/mol
C����Ҫ���Ȳ��ܷ����ķ�Ӧһ�������ȷ�Ӧ
D��1mol����ȼ��������̬ˮ�Ͷ�����̼���ų��������Ǽ����ȼ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijѧ����������װ��̽����Ȫʵ�顣����A��F�ֱ�Ϊ����������ķ���װ�ã�CΪ������������������ⷴӦ��װ�á�
��
��ش��������⣺
��1��װ��F�з�����Ӧ�Ļ�ѧ����ʽ ��
��2��װ��A�еķ�Һ©����Һ��a��ѡ�� ��ѡ������ѡ��Ĵ��ţ�
A������ B��Ũ���� C��ϡ���� D��ϡ����
��3�����߿���Ӧ���ӱ�Ҫ�ij���װ�ã������ͼ�ġ���ѡװ�á���ѡ�����װ�õı�ţ��������пո�B__________��D__________��E__________��
��4����K1��K2��������ѹ�µ�H2S��Cl2�������1:1������ƿ����ƿ�з����ķ�Ӧ�û�ѧ����ʽ��ʾΪ ���ر�K1��K2��ʼ�տ�������ƿ�ڲ�������Ȫ�����������ǣ� ��
��5���ڲ�����4���Ļ����ϣ�������Ȫ���������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���б�������ȷ����
A��NaHS��ˮ�еĵ��뷽��ʽΪ��NaHS=Na++HS- ��HS- =H++S2-
B��ͬ���ʵ���Ũ�ȵİ�ˮ�����ᷴӦ������ʱ���������V��NH3��H2O����V��HCl��
C��Na2SO3��Һ�У�c��H+��+ c��HSO3-��+ 2c��H2SO3��= c��OH-��
D��ͬŨ�ȵ�������Һ�У�c ��CH3COO-���Ĵ�С��CH3COONa��CH3COONH4��CH3COOH
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и��������У���Ϊͬ���칹�����
A������ˮ B�������ͳ��� C��1H��3H D��CH3CH20H��CH30CH3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������Һ�У��ܴ����������������
A��K+��OH-��S042- B��Cl-��N03-��Cu2+
C��Al3+��C032-��Na+ D��Ca2+��HC03-��NH4+
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����и��������У���ѧ����������ȫ��ͬ����
����HI��NaI �£�H2S��CO2 �ã�F2��NaBr �ģ�Cl2��CCl4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������Ԫ�ص�����aXm����bYn����cZn����dRm��(a��b��c��dΪԪ�ص�ԭ������)�����Ǿ�����ͬ�ĵ��Ӳ�ṹ����m��n����������������ж���ȷ���ǣ�����
��a��b��m��n����Ԫ�ص�ԭ������a��b��c��d��
��Ԫ�طǽ�����Z��R��������������Ӧˮ����ļ���X��Y
A���ڢۡ����������� B����
C���٢ڢۢ� D���٢ڢ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com